Test_Bank_For_Finite_Mathematics_for_Business_Economics_Life_Sciences
$ 15

CCHT Practice Questions (CCHT Practice Tests & Exam Review for the CCHT Exam) Part 1 (unanswered)
$ 10

GCE Mathematics A H240/01: Pure Mathematics Advanced GCE Mark Scheme for November 2020
$ 7.5
.png)
WGU C779 Web Development Foundations Questions and Answers Graded A+
$ 8

Pearson Edexcel Mark Scheme (Provisional) Summer 2021 Pearson Edexcel International GCSE In Science (Single Award) (4SS0) Paper 1B
$ 6

A-level DESIGN AND TECHNOLOGY: PRODUCT DESIGN 7552/2 Paper 2 Designing and Making Principles Mark scheme June 2022
$ 8

GCE Further Mathematics B MEI Y422/01: Statistics major A Level Mark Scheme for June 2023
$ 4

OCR A LEVEL JUNE 2022 DESIGN AND TECHNOLOGY PAPER 2 RESOURCE BOOK - PROBLEM SOLVING IN PRODUCT DESIGN
$ 1

OCR A LEVEL JUNE 2022 DESIGN AND TECHNOLOGY PAPER 2 RESOURCE BOOK - FASHION AND TEXTILES.
$ 1

Quiz 3: Unified Modeling Language (UML)
$ 8.5

OCR GCE Further Mathematics A Y533/01: Mechanics Advanced Subsidiary GCE Mark Scheme for Autumn 2021
$ 12

Solution Manual For Data Analytics for Accounting, 3rd Edition Richardson Chapter 1-9
$ 9.5

H006-01 OCR AS LEVEL 2022 DESIGN AND TECHNOLOGY MARK SCHEME PAPER 1
$ 3

eBook Web Scraping with Python Data Extraction from the Modern Web 3rd Edition By Ryan Mitchell
$ 30
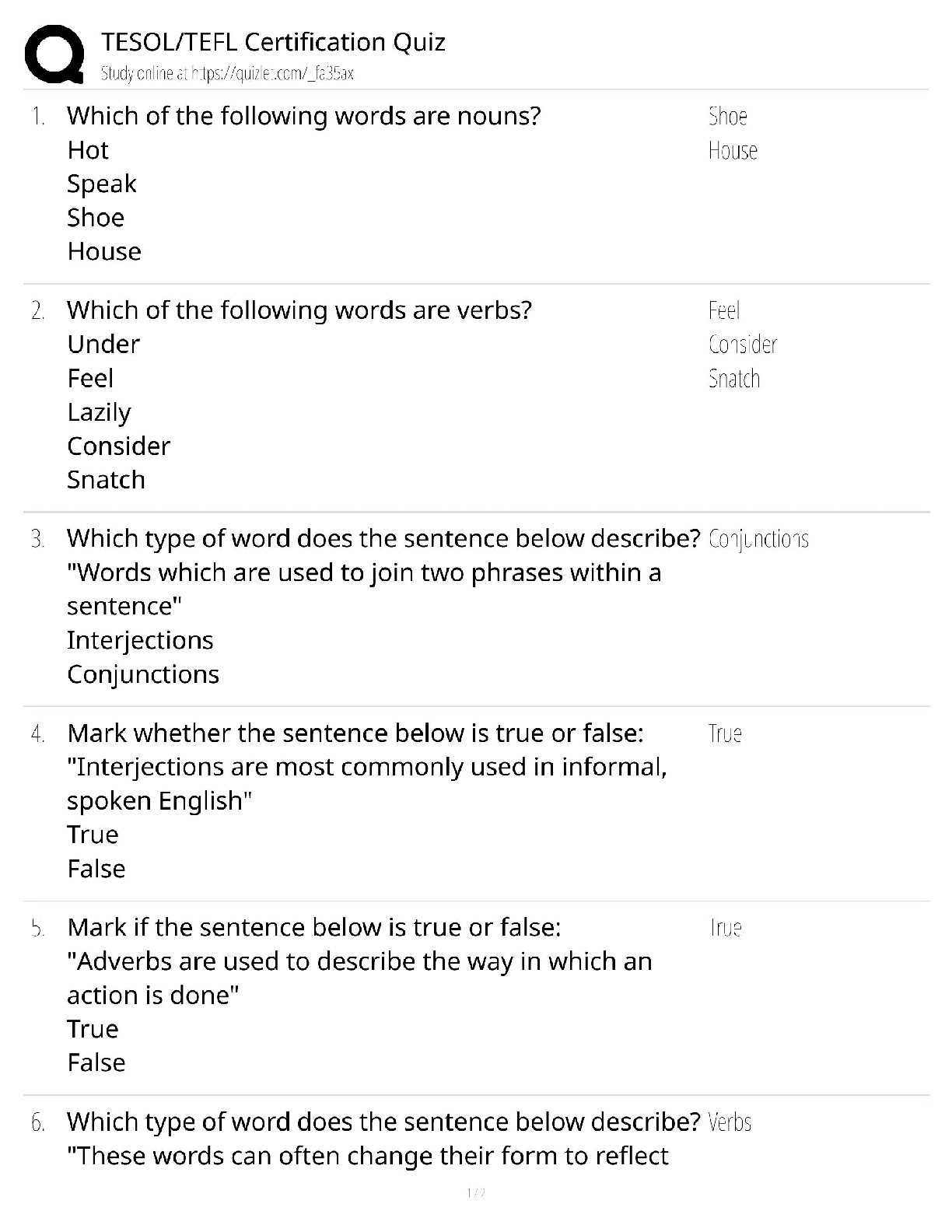
TESOL TEFL Certification Quiz / 120-Hour Course Test Bank / 2025 Update / Score 100%
$ 18

TRANSACTION COMPS MODELLING WALL STREET PREP EXAM NEWEST 2025 ACTUAL EXAM ALL 100 QUESTIONS AND CORRECT DETAILED ANSWERS WITH RATIONALES (VERIFIED ANSWERS) |ALREADY GRADED A+
$ 10.5

Test Bank for Business Mathematics In Canada 10th edition F. Ernest Jerome, Tracy Worswick
$ 9
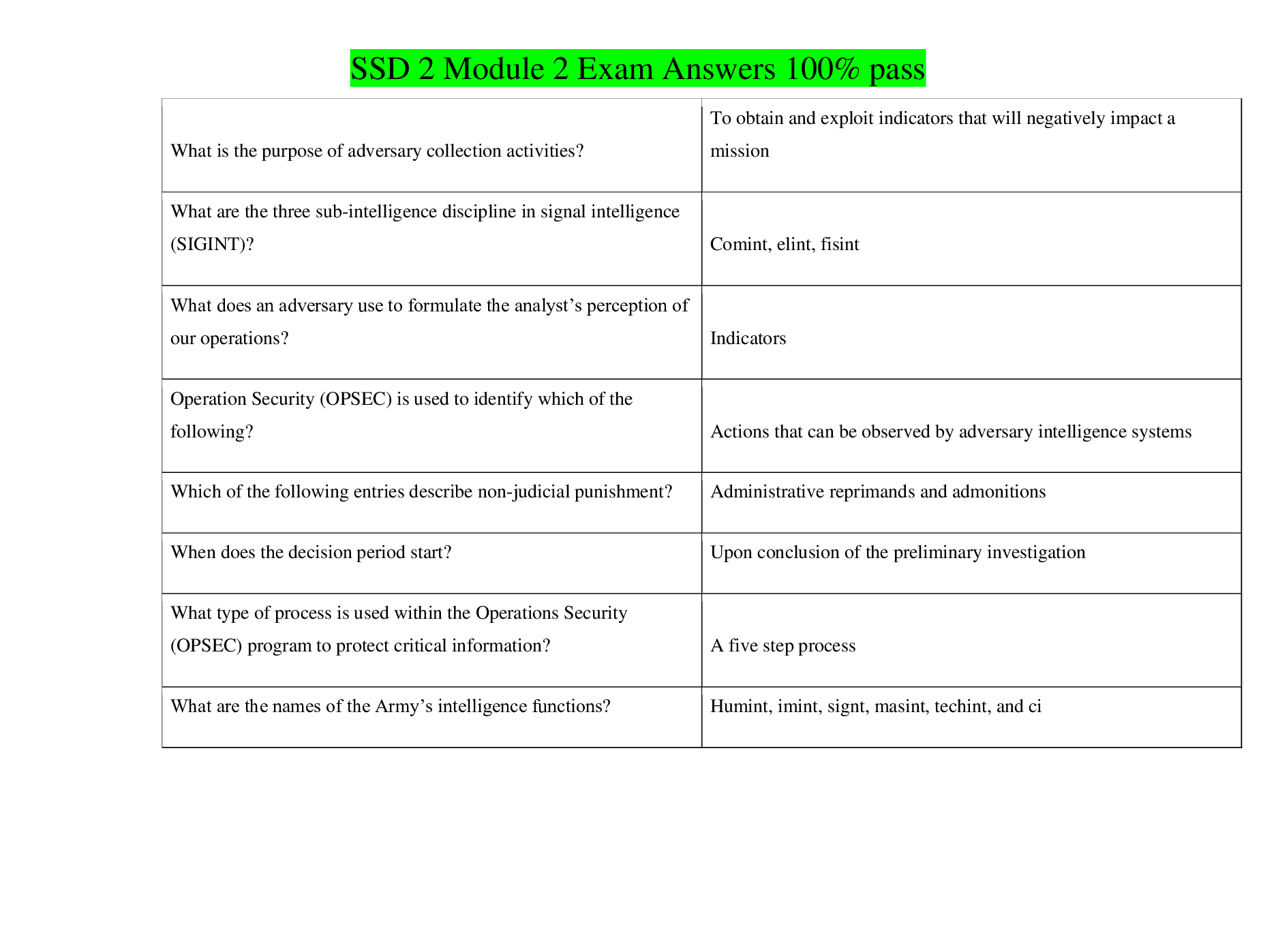
SSD 2 Module 2 Exam Questions with Correct Answers 100% pass
$ 9

OCR A Level Geology H414-01 Fundamentals of geology QUESTION PAPER Monday 6 June 2022 – Afternoon
$ 16

DATABASE 0047relational_database_unit_2_milestones_2 UNIT 2 — MILESTONE 2 Score 17/25 | Western Governors University

.png)


.png)
.png)




.png)
.png)