ioethical standards 1) autonomy-the right to choose for himself or herself 2) freedom3) veracity-right to truth 4) privacy-the right of privacy avoids conflict and promotes harmony 5) beneficence-actions performed that c
...
ioethical standards 1) autonomy-the right to choose for himself or herself 2) freedom3) veracity-right to truth 4) privacy-the right of privacy avoids conflict and promotes harmony 5) beneficence-actions performed that contribute to the welfare of others 6) fidelity-right to what has been promisedMedical Applications Apps Providing Access to Electronic Copies Apps for General Patient Education Generic Aids or General Purpose Apps Apps as Educational Tools Apps Automating Office OperationsSome mobile apps may meet the definition of a medical device but because they pose a lower risk to the public, the FDA intends to exercise enforcement discretion over these devices (meaning it will not enforce requirements under the FD&C Act). One example is a mobile app that makes a light emitting diode (LED) operate. If the manufacturer intends the system to illuminate objects generally (i.e., without a specific medical device intended use), the mobile app would not be considered a medical device. If, however, through marketing, labeling, and the circumstances surrounding the distribution, the mobile app is promoted by the manufacturer for use as a light source for providers to examine patients, then the intended use of the light source would be similar to a conventional device such as an ophthalmoscope.FDA Oversight for Medical Devices The Food and Drug Administration (FDA) (2013) recognizes the extensive variety of actual and potential functions of mobile apps, the rapid pace of innovation in mobile apps, and the potential benefits and risks to public health represented by these apps. The FDA intends to apply its regulatory authorities to select software applications intended for use on mobile platforms. Given the rapid expansion and broad applicability of mobile apps, the FDA is issuing this guidance document to clarify the subset of mobile apps to which the FDA intends to apply its authority.
[Show More]








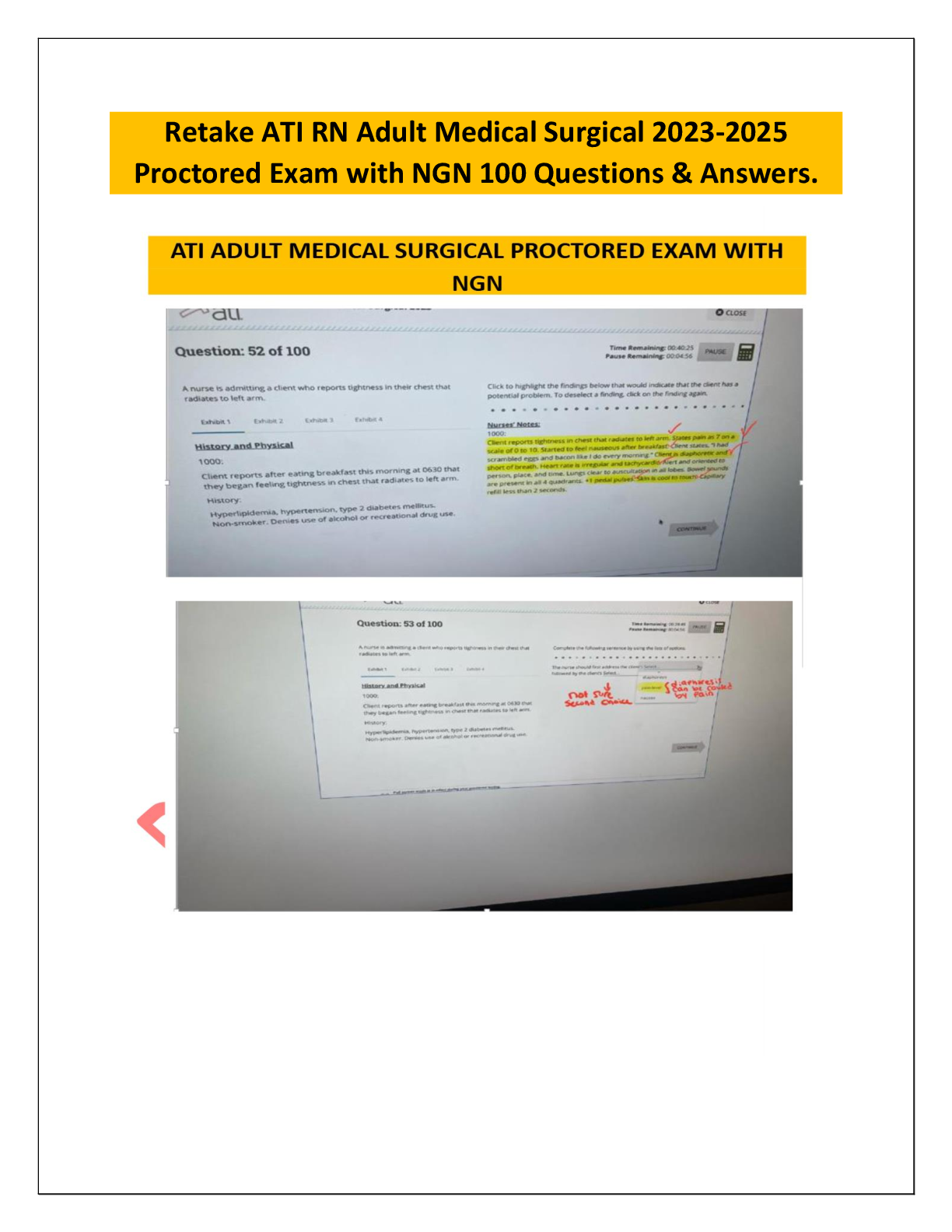






 JN21.png)




