There are two major classes of compounds typically encountered as part of an introductory course: ionic and covalent
compounds. The concepts describing how these compounds are held together can be developed as you progr
...
There are two major classes of compounds typically encountered as part of an introductory course: ionic and covalent
compounds. The concepts describing how these compounds are held together can be developed as you progress
through your studies. However, before you get to those concepts you must be able to quickly classify a compound into
one class or the other. In other words, your ability to classify compounds will guide how you will think about bigger
ideas.
The Model:
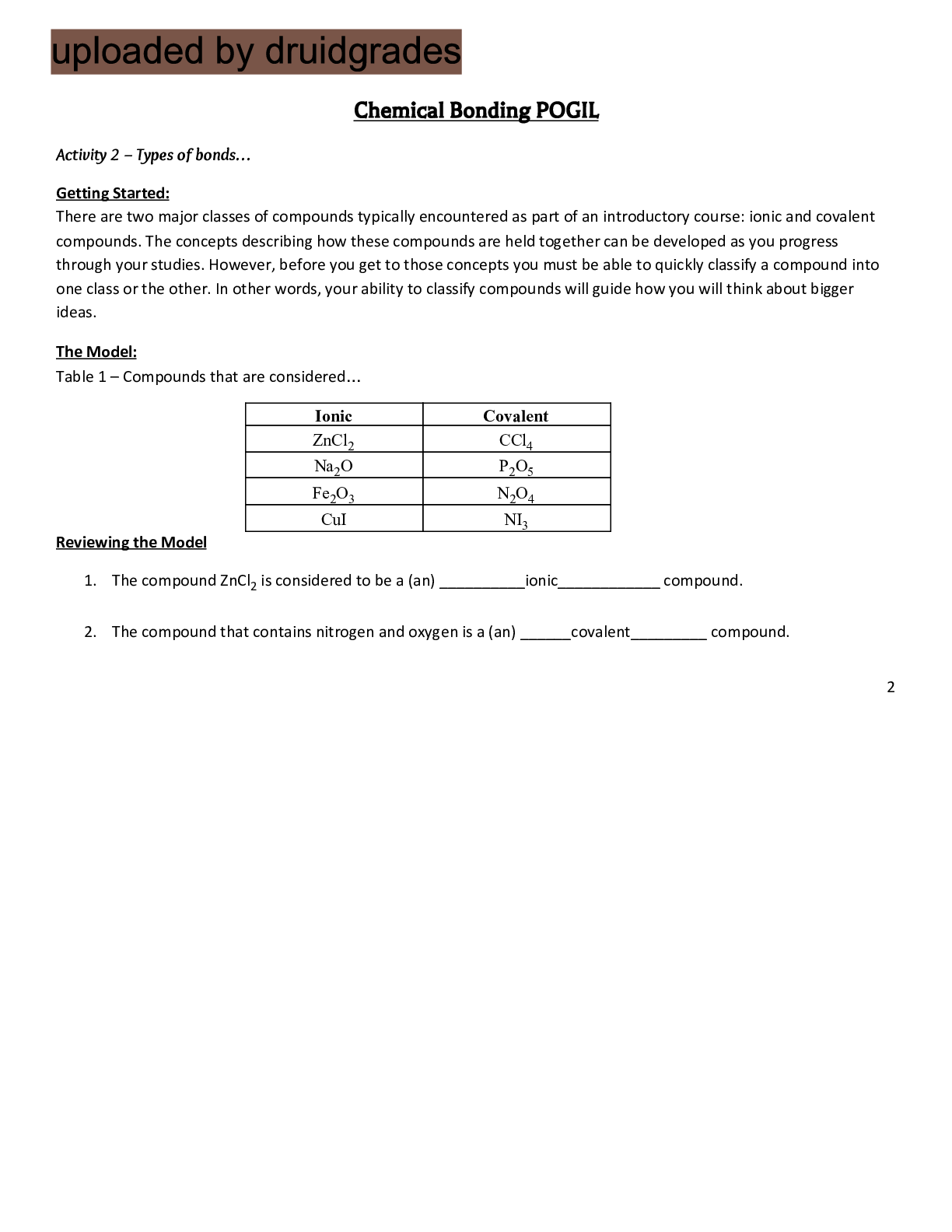
Table 1 – Compounds that are considered…
Reviewing the Model
1. The compound ZnCl2
is considered to be a (an) __________ionic____________ compound.
2. The compound that contains nitrogen and oxygen is a (an) ______covalent_________ compound.
2
Ionic Covalent
ZnCl2 CCl4
Na2O P2O5
Fe2O3 N2O4
CuI NI3
uploaded by druidgrades
Exploring the Model
3. Does the classification seem to be made based on how many atoms of each element are represented in the
formula?
4. Write the symbols for the elements presented by the Model (Table 1) near their correct location on the
outline of the periodic table.
5. Compare the types
of elements found (metals or nonmetals) for the two classes of compounds. Do you see any trend in the type of
elements present and the classification?
no it doesn’t
ionic is metal and nonmetal
covalent is non metal, metal
Exercising Your Knowledge
6. Classify each of the following as either ionic or covalent.
a. NaBr
____ionic_____________
___ f. BaS
__________Ionic_______
___
b. SF6
_______covalent_______
______ g. CsF2
ionic
c. CoBr2
_____ionic____________
___ h. CrCl3
ionic
d. OF2
____covalent__________
______ i. CO2
__covalent____________
______
e. NO2
_____covalent_________
______ j. CO
covalent_
[Show More]


.png)