MECH 6501
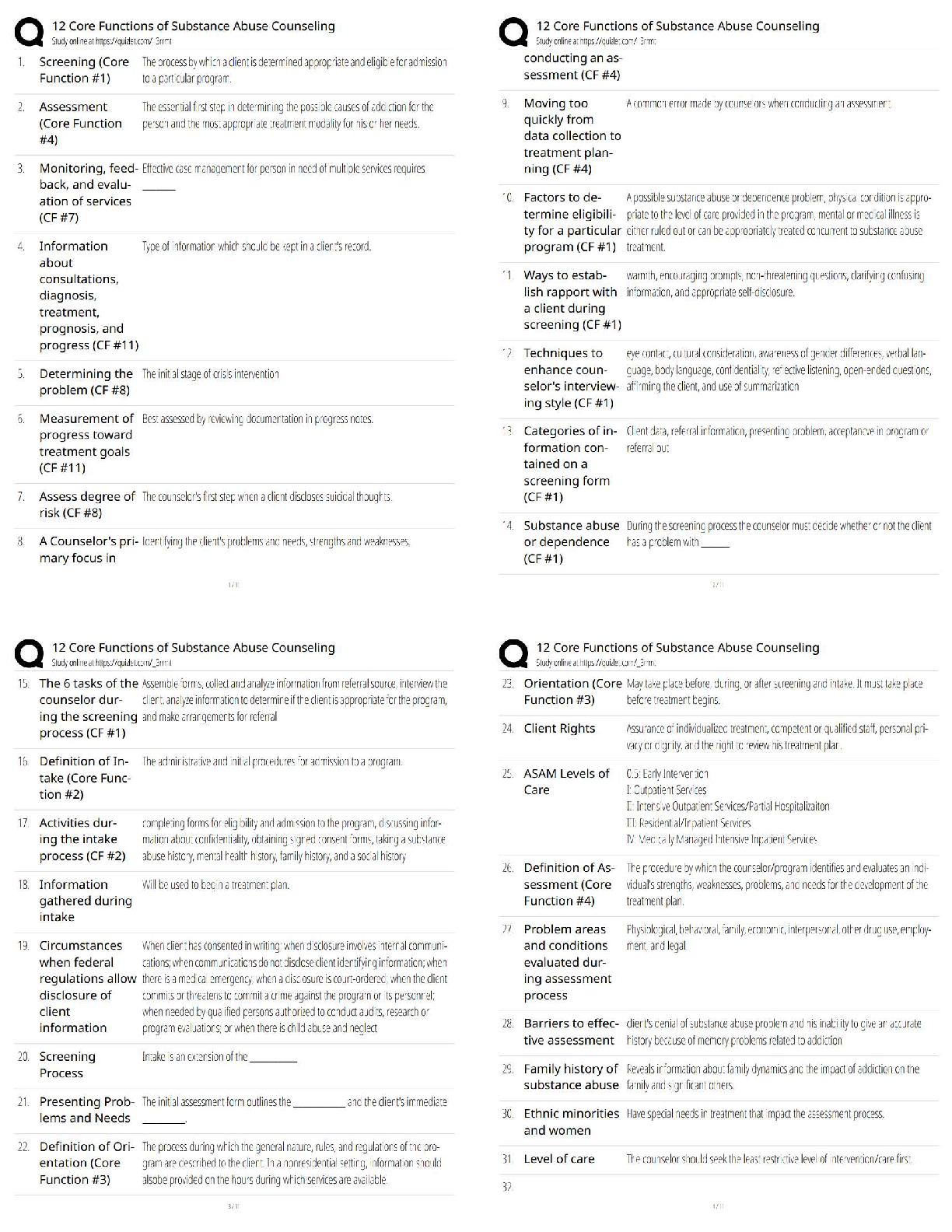
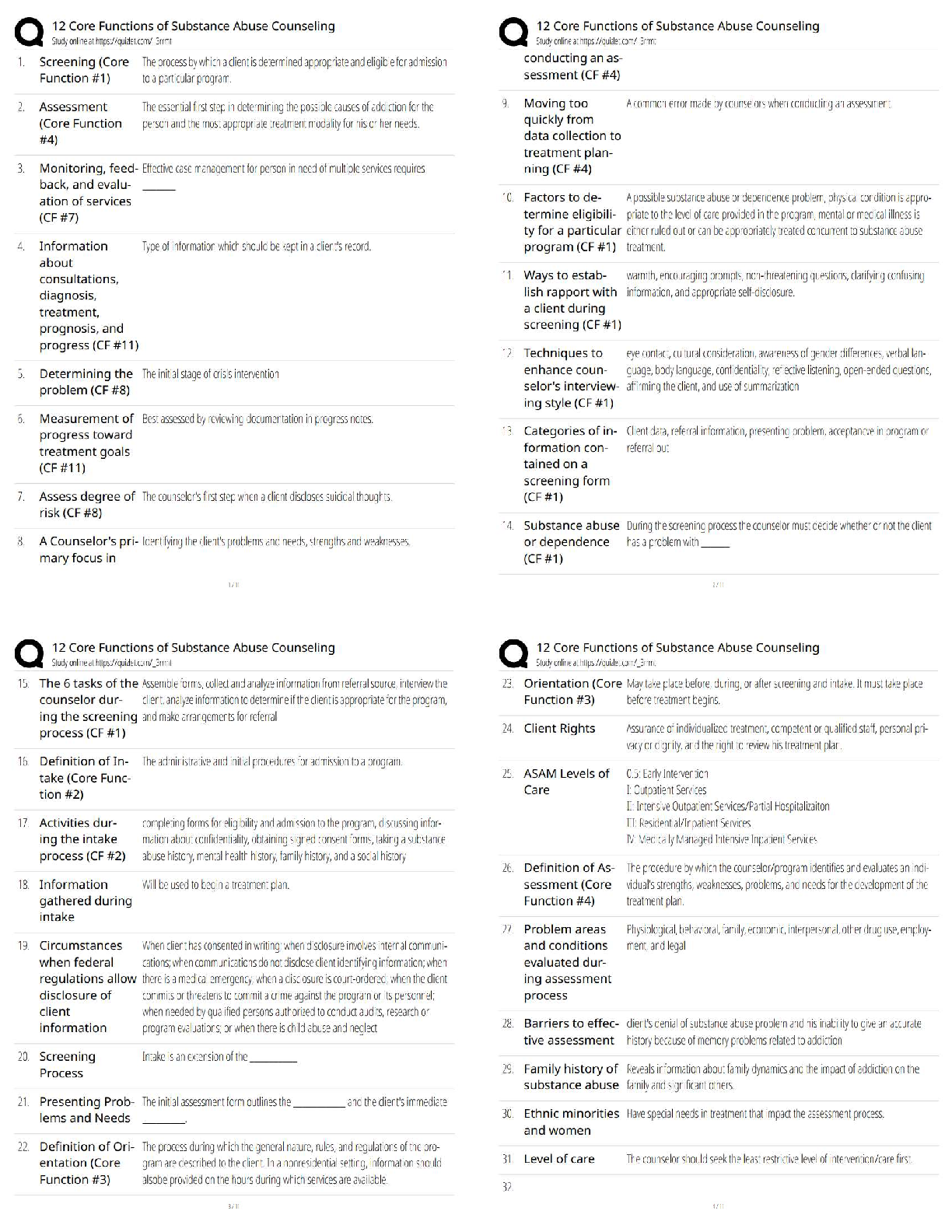
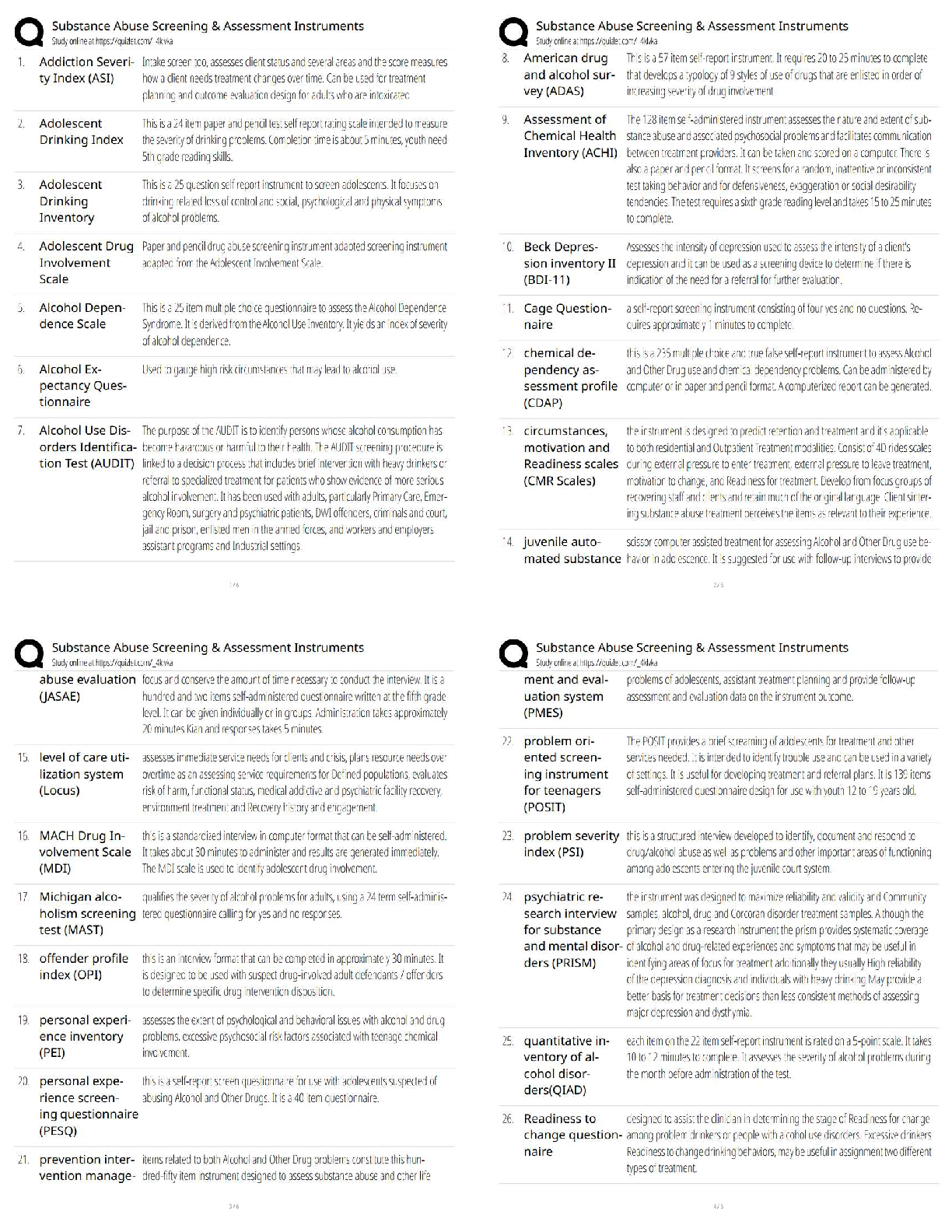
Assignment 1
1. Calculate the minimum radius ratio for an AB ceramic compound with a coordination
number of four assuming a hard sphere model for both the cations and anions.
2. Give the expected coordinati
...
MECH 6501
Assignment 1
1. Calculate the minimum radius ratio for an AB ceramic compound with a coordination
number of four assuming a hard sphere model for both the cations and anions.
2. Give the expected coordination number of both the anions and cations, taking into
account charge balance, for the following ceramics: a) ZrO2 b) MgO and c) BeO. Explain
any differences between the calculated and actual observed CNs.
3. For the above oxides, calculate the percent ionic/covalent nature of these ceramics using
Pauling's scale and briefly outline the implication of these values on the general
properties of these ceramics.
4. Li2O forms with the antifluorite structure. Sketch this structure labelling the ions. Sketch
the following planes of this structure indicating those ions which appear on the particular
plane: a) (100), b) (110) and (004). Provide the coordinates of the ions where appropriate.
5. Calculate the crystallographic density of CaO, where the atomic masses of Ca and O are
40 and 16, respectively (Avogadro's number is 0.602 x 1024 atoms mol-1). What
assumptions are made? The measured density of CaO is 3.25 g cm-3. Explain the
difference between your calculated value and this value.MECH 6501
Assignment 1 (Solutions)
1. Calculate the minimum radius ratio for an AB ceramic compound with a
coordination number of four assuming a hard sphere model for both the cations
and anions.
2. Give the expected coordination number of both the anions and cations, taking into
account charge balance, for the following ceramics: a) ZrO2 b) MgO and c) BeO.
Explain any differences between the calculated and actual observed CNs.
3. For the above oxides, calculate the percent ionic/covalent nature of these ceramics
using Pauling's scale and briefly outline the implication of these values on the
general properties of these ceramics.
4. Li2O forms with the antifluorite structure. Sketch this structure labelling the ions.
Sketch the following planes of this structure indicating those ions which appear
on the particular plane: a) (100), b) (110) and (004). Provide the coordinates of
the ions where appropriate.
5. Calculate the crystallographic density of CaO, where the atomic masses of Ca and
O are 40 and 16, respectively (Avogadro's number is 0.602 x 1024 atoms mol-1).
What assumptions are made? The measured density of CaO is 3.25 g cm-3.
Explain the difference between your calculated value and this value.
[Show More]





.png)

.png)
.png)
.png)


.png)