FUEL SCIENCE
PROBLEM SET #3
The usual hints, instructions, terms and conditions apply.
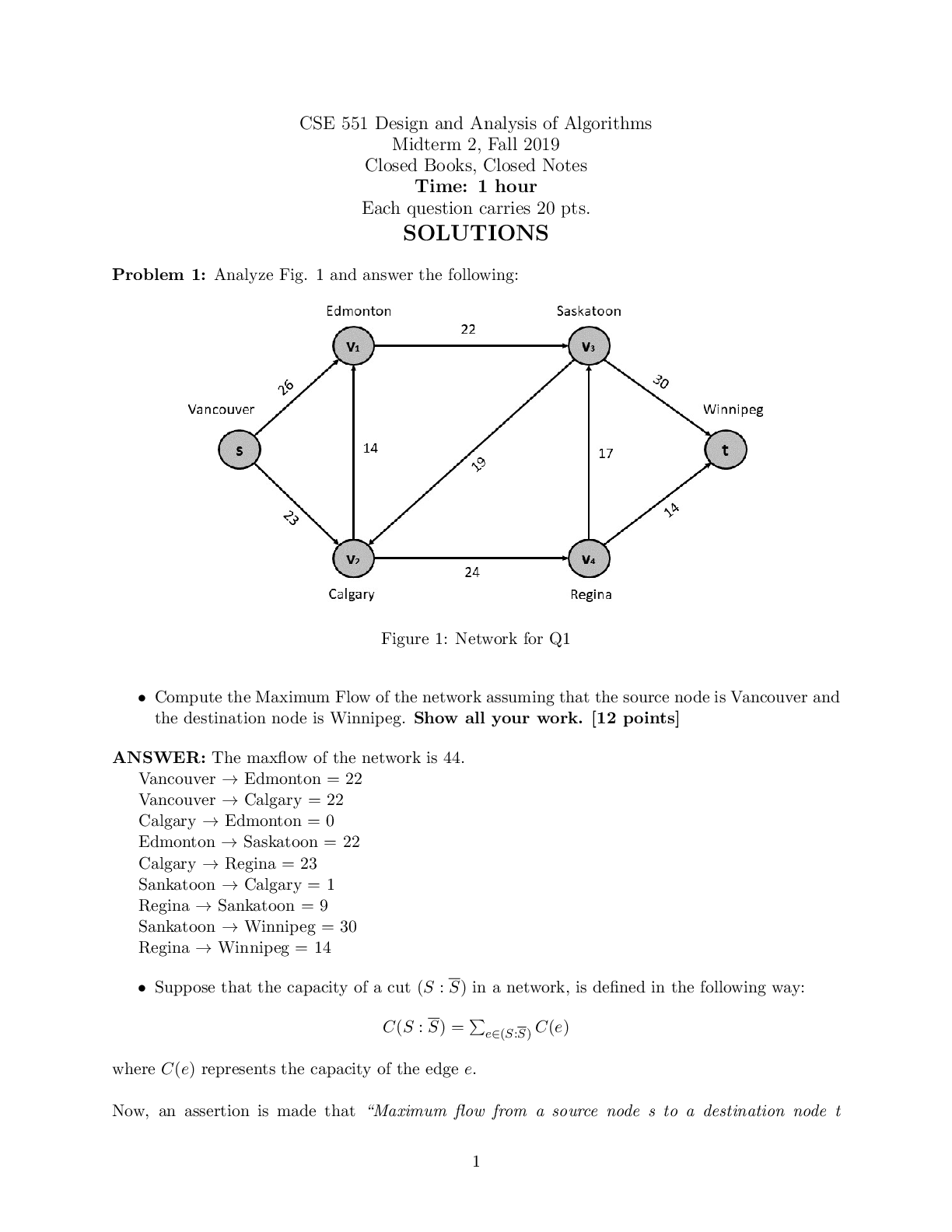
1. In 2004 (the last year for which complete data are yet available) there was a price
difference of $10.55 per barrel between pe
...
FUEL SCIENCE
PROBLEM SET #3
The usual hints, instructions, terms and conditions apply.
1. In 2004 (the last year for which complete data are yet available) there was a price
difference of $10.55 per barrel between petroleum with API ≥ 45° and that with API ≤
20°. (a) Why would refiners pay a price premium based on API gravity? In particular,
why can a 45° API crude command a higher price than, say, a 20° crude? (b) Of these
two oils, which is the more likely to give problems with coke deposition in thermal or
catalytic processes? Why? (c) Which would more likely benefit from being processed
through a visbreaker before being sent further through the refinery? Why? (d) And, in
going through distillation, which is likely to give the lower yield of resid? Why?
a) The higher API the higher amount of gasoline can be produced.
b) Due to its high concentration of heavies in the crude, the 20° is more likely to give
problems with coke deposition.
c) If the 20° was placed through a visbreaker it would increase the amount of gasoline
obtained from the refining process.
d) The 45° because of its low concentration of small hydrocarbon molecules
2. Suppose that you have the job assignment of selecting catalyst for an FCC unit.
Suppose further that you can select from a variety of available catalysts that have
different Si/Al ratios: Catalyst A has Si > Al, catalyst B has Si ≈ Al, and catalyst C has Si
< Al. (a) Which catalyst would you specify, and why, if the FCC feedstock consists
almost entirely of alkanes? (b) Answer the same questions if there were abundant alkenes
in the feedstock.
a) Catalyst C would be the best in this case because of the high concentration of alkanes
in the feedstock.
b) Catalyst A
3. Classify each of the following as a Lewis acid or a Brønsted acid: (a) HClO4, (b) BF3,
(c) AlH3, (d) H2S, (e) HCO3–, (f) SO3, (g) HF, (h) H+ (think!), (i) ZnCl2, (j) H3PO4.
A
Bronsted
F Lewis
B
Lewis
G Bronsted
C
Lewis
H Lewis
D
Bronsted
E
Bronsted
J Bronsted
4. (a) Why is coking of the catalyst inevitable in an FCC unit? Explain in terms of the
“inverted V” diagram we first used to discuss kerogen maturation. (b) If process control
is not adequate, it is possible sometime to “over-crack” the feed, and get high yields of
gases instead of gasoline. If this happens, will catalyst coking be worse or better than
during normal operation? Why?
a) Coke is produced from carbon rich molecules. As hydrogen is transferred from and to
the crude oil, molecules become rich in hydrogen and carbon. This transfer of Hydrogen
molecules is what causes coking.
b) It would be better than normal operation, because over-cracking decreases the amount
of residue left over from the process.
[Show More]
, Questions and Answers, All Correct Study Guide, Download to Score A.png)