MSE 2010 Midterm 1 Material
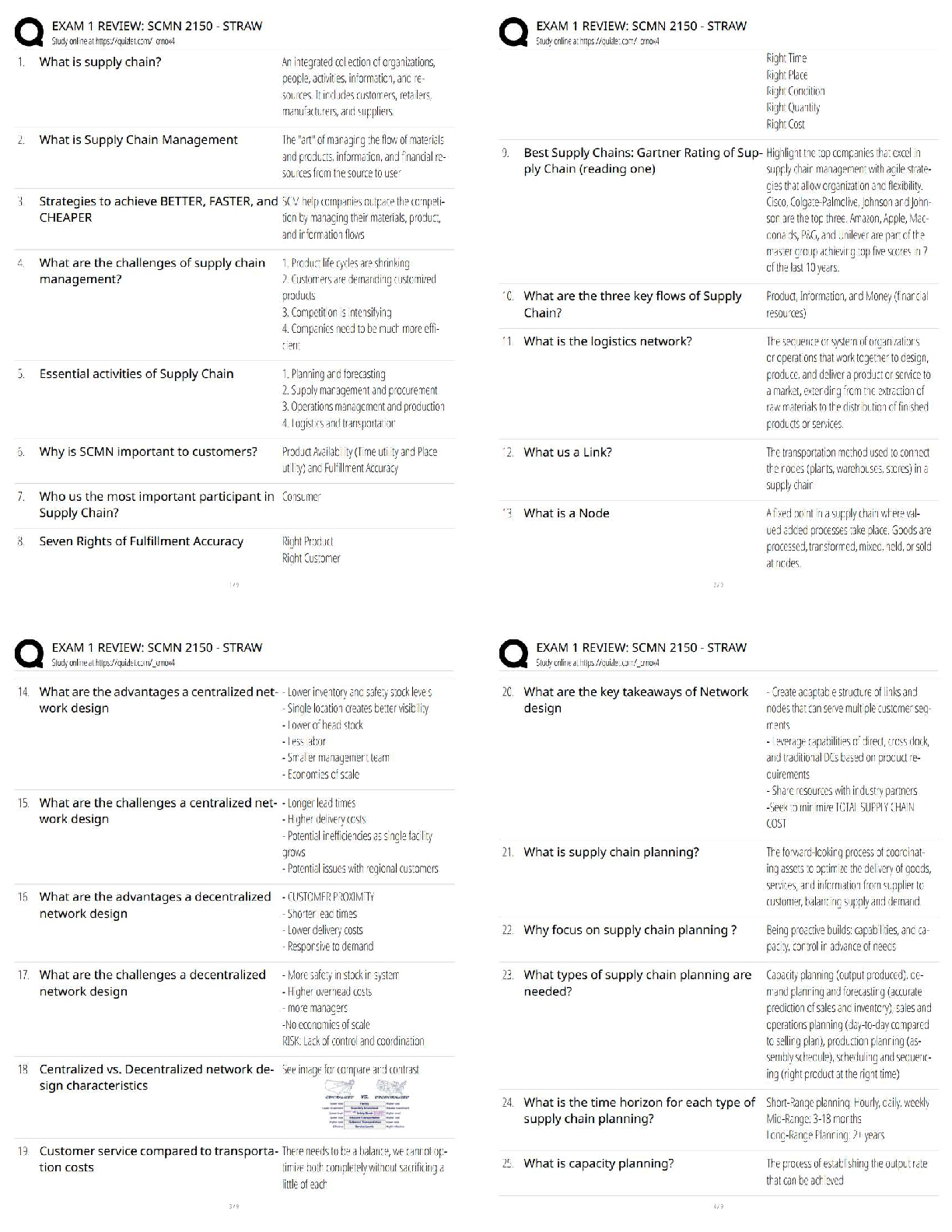
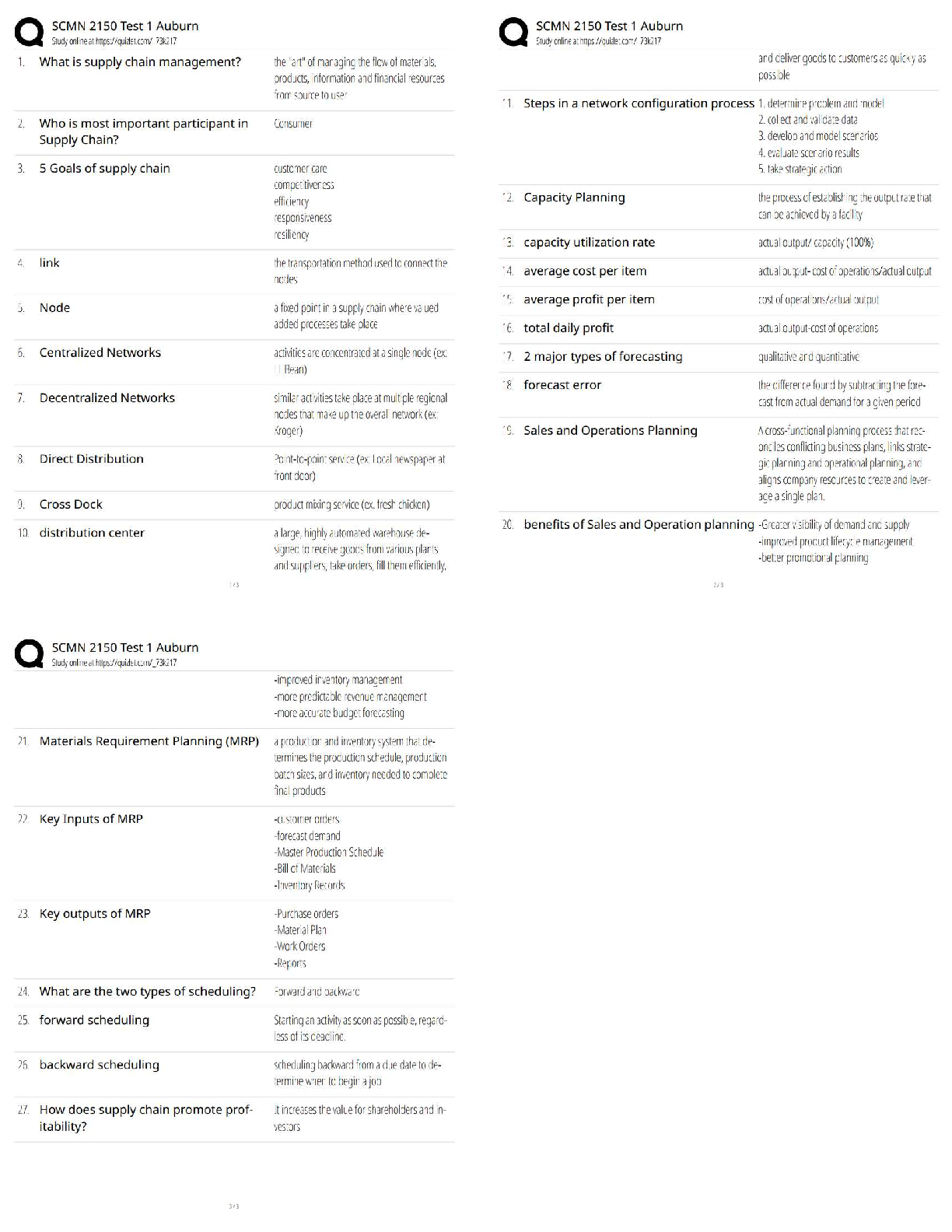
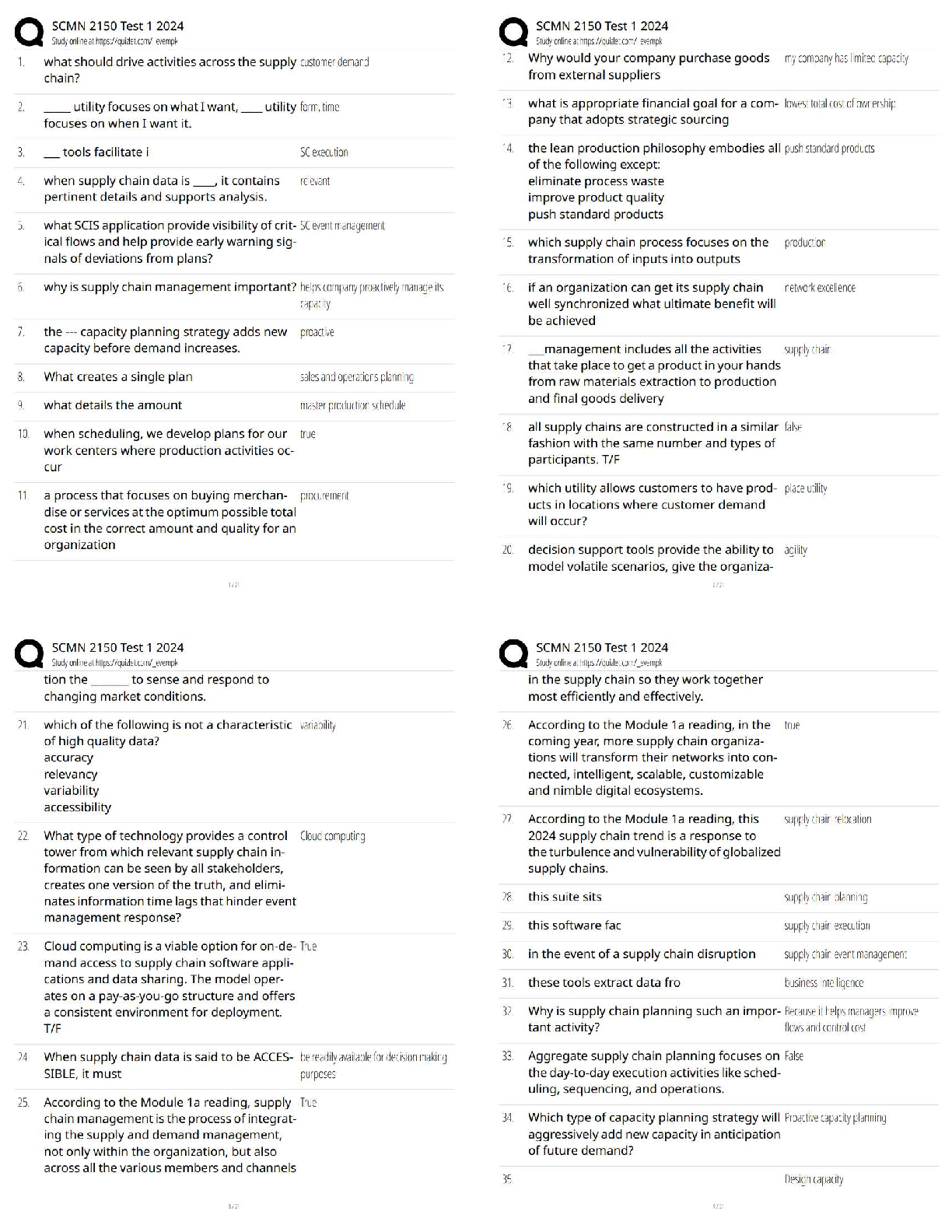
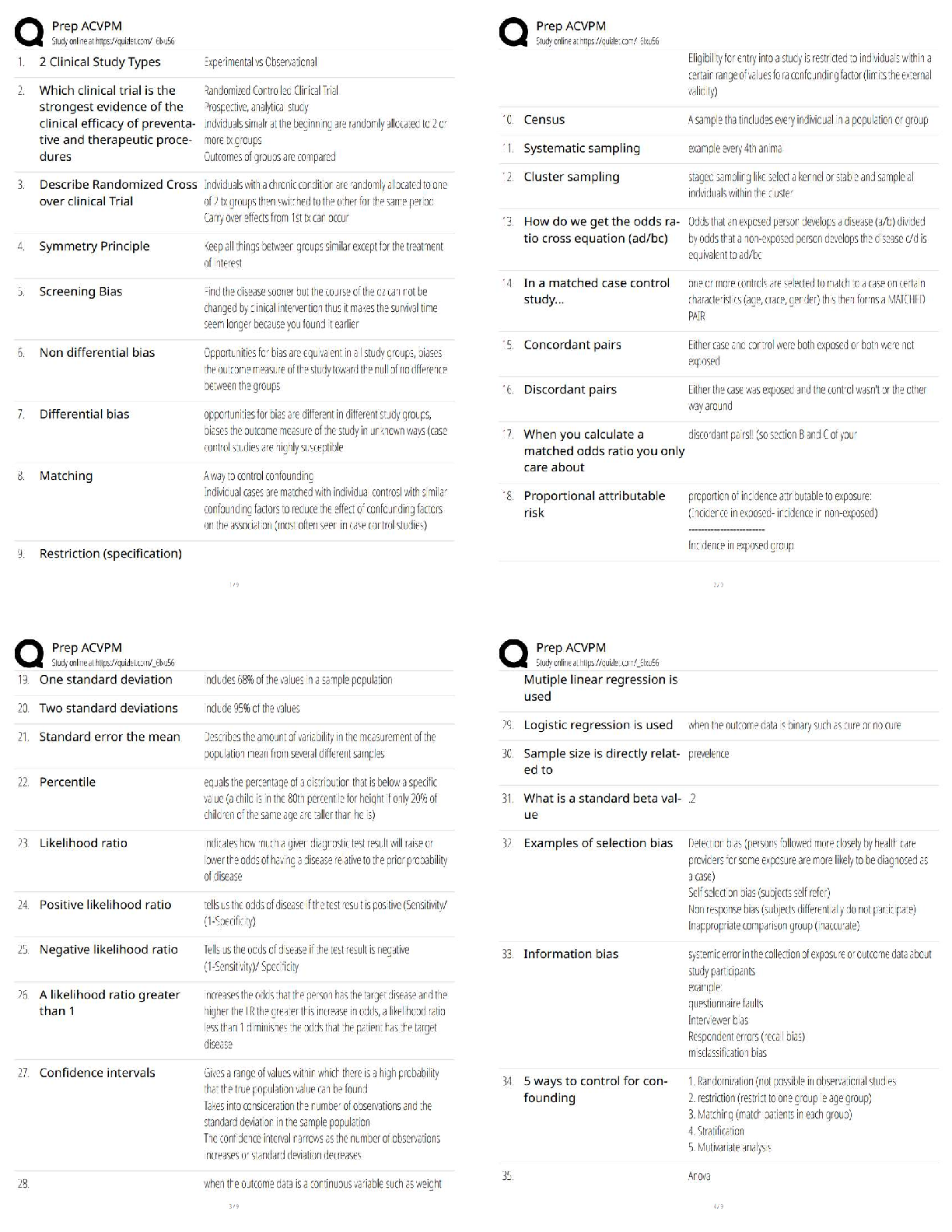
A - ✔✔Which of the following best characteristics describes a metallic bond?
A. A "sea of electrons".
B. Directional
C. Secondary bond type
D. Exchange of electrons from one atom to anoth
...
MSE 2010 Midterm 1 Material
A - ✔✔Which of the following best characteristics describes a metallic bond?
A. A "sea of electrons".
B. Directional
C. Secondary bond type
D. Exchange of electrons from one atom to another
B - ✔✔Which of the following best characteristics describes a covalent bond?
A. A "sea of electrons".
B. Directional
C. Secondary bond type
D. Exchange of electrons from one atom to another
B - ✔✔In which one of the following major classes of materials do van der Waals bonding play
a major role in determining the physical properties of the material?
A.Metal
B.Polymer
C.Ceramic
D.Semiconductor
B - ✔✔What type of bonds are broken when water boils?
A.metallic
B.secondary
C.ionic
D.covalent
A - ✔✔For pure H2O at atmospheric pressure, which of the following is/are true?
A.It solidifies at a specific temperature
B.It solidifies over a range of temperatures
C.Both the liquid and solid phases are stable over a range of temperatures.
C - ✔✔The melting points of most plastics are lower than most metals because:
A. Covalent bonds are weaker than metallic bonds.
B. Ionic bonds are weaker than metallic bonds.
C. Van der Waals bonds are weaker than metallic bonds.
D. Covalent and Van der Waals bonds are weaker than metallic bonds.
E. Ionic and Van der Waals bonds are weaker than metallic bonds.
B - ✔✔What are the units for the coefficient of linear thermal expansion (alpha)?
[Show More]