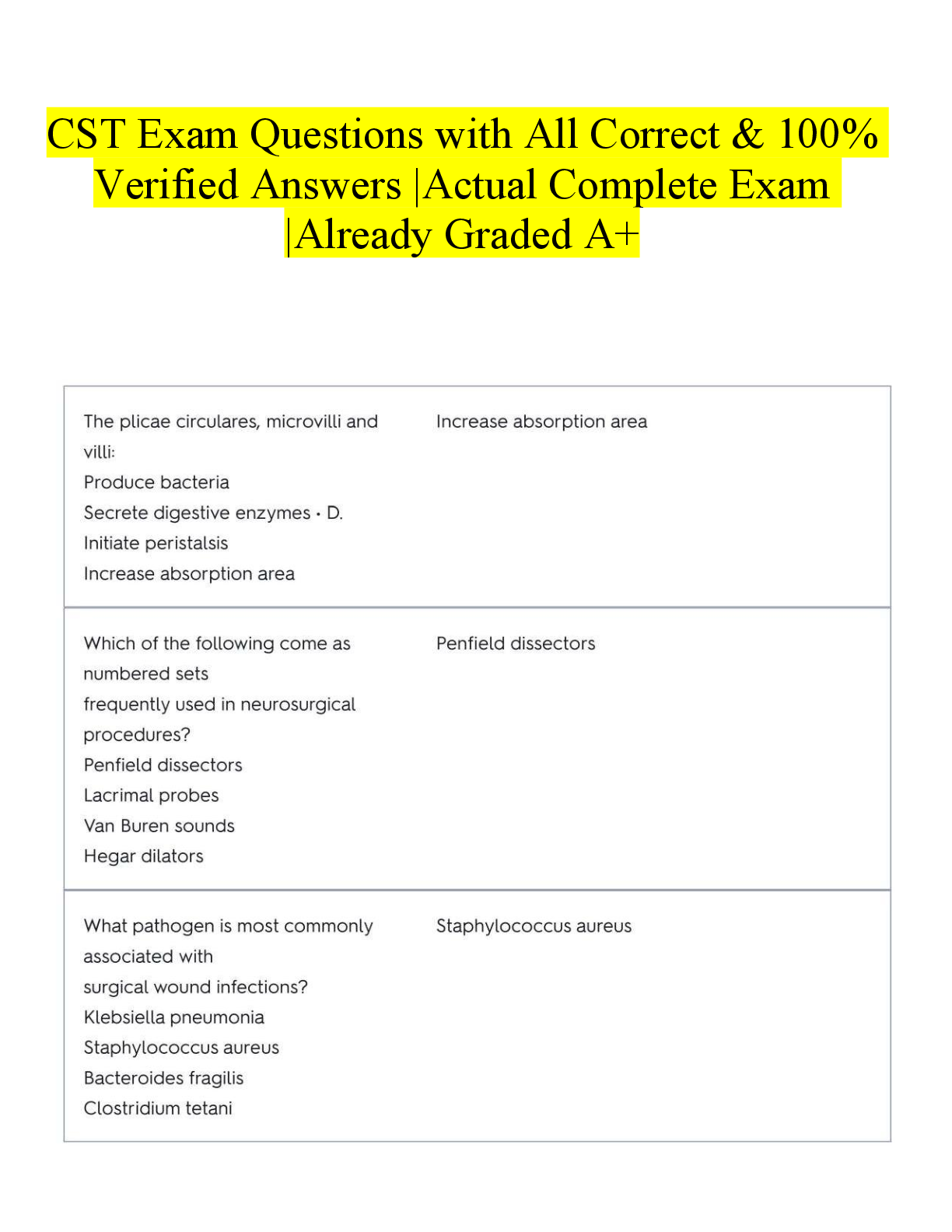
Military Science > EXAM > SCIN131 Lesson 05 - Week 05 Quiz (All)
SCIN131 Lesson 05 - Week 05 Quiz
Document Content and Description Below
Question 14 of 25 `Suppose you were tasked with producing some nitrogen monoxide (a.k.a. nitric oxide). I'm sure this is often requested of you. You can do it by combusting ammonia (be careful!). Th ... e equation would be as follows: 4NH3 + 5O 2→ 4NO + 6H2O. If you form 3.50 mol of water, how much NO forms? A. 2.33 mol. B. 4.00 mol. C. 4.50 mol. D. 6.75 mol. E. none of the above Feedback: Good. See section 8.6 Question 15 of 25 `Methane is also known as natural gas, and its combustion is a very common reaction: CH4 + 2 O2 → CO2 + 2 H2O. Remember that we can use stoichiometry if we know the correct coefficients. So, which of these conversion factors will NOT work for this reaction? NOTE: All numbers located immediately after elemental symbols should be considered subscripts. A. 1 mole O2 = 32.00 g B. 2 mole H2O = 18.02 g C. 2 mole O2 = 1 mole CO2 D. 1 mole CH4 = 2 moles H2O E. none of the above [Show More]
Last updated: 3 years ago
Preview 1 out of 11 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$7.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Aug 12, 2021
Number of pages
11
Written in
All
Additional information
This document has been written for:
Uploaded
Aug 12, 2021
Downloads
0
Views
47