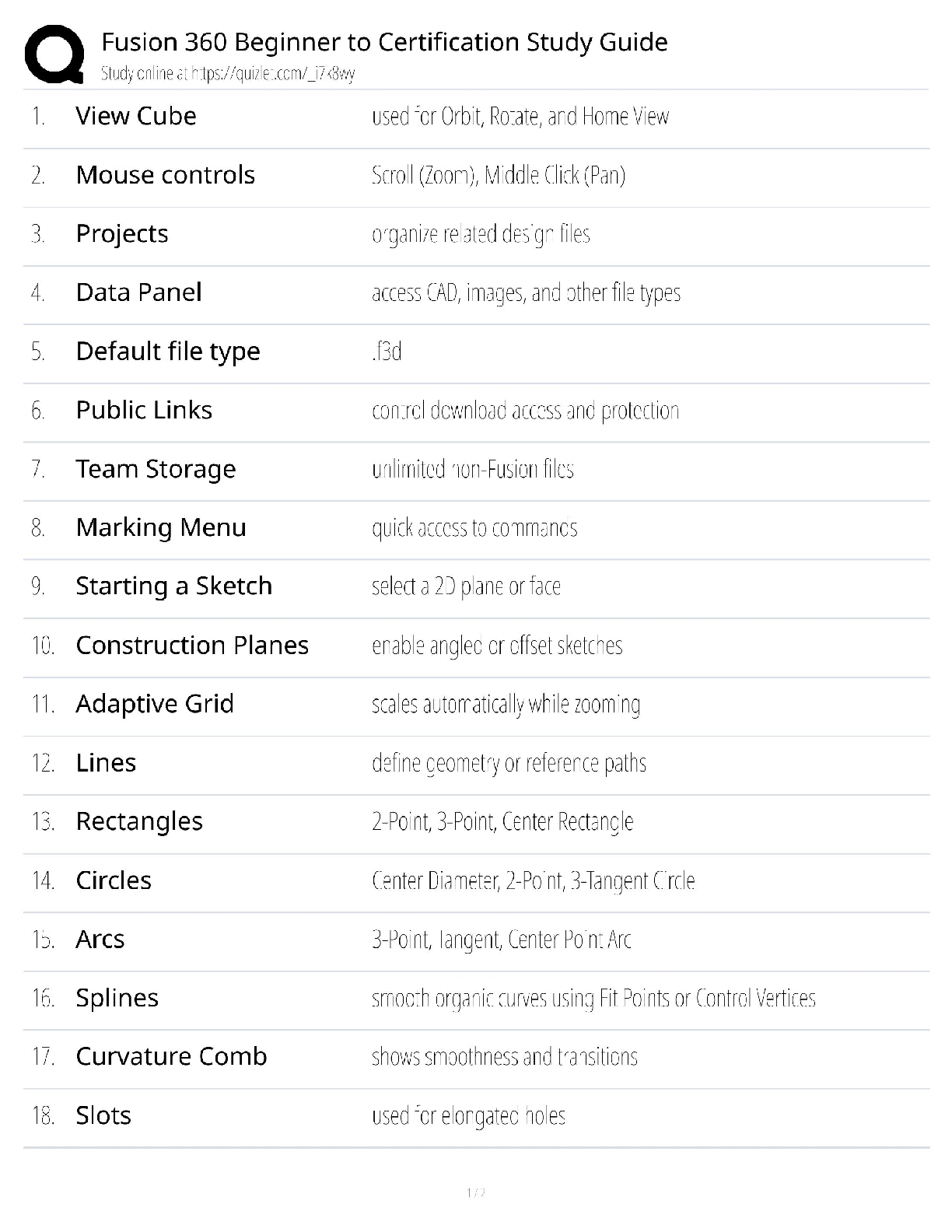
Fusion 360 Beginner to Certification Study Guide / Score 100% / New 2025 Update / Autodesk Certified User Exam Prep
$ 12

HSAD 331 NON PROFITS AND HEALTH CARE EXAM Q & A WITH RATIONALES 2024
$ 15

ENGL 147N Week 6 Assignment: What Does the Opossum Say Invent a Scenario Involving a Genre of Argument (Presentation)
$ 10

NEIEP 800 Final Review Questions & Answers With Complete Solution
$ 8.5
 Course Notes(ANF413).png)
Corporate and Business paper F4 ( English ) Course Notes(ANF413) For Better Grades
$ 10

Pathways to Astronomy 6th Edition By Steven Schneider, Stephen Schneider, Thomas Arny (SOLUTIONS MANUAL]
$ 25

WGU D115 OA Study Guide Unit 7 Verified 100% Questions 2024
$ 19.5

TI F.P 100,23 Agile Exam Questions and Answers. This Document Contains 65 Questions and Answers.
$ 10

HESI MEDSURGE BRAND NEW QUESTIONS WITH NGN STYLE 55 Q&A V1 2023-2024
$ 22.5

eBook Introduction to Combinatorial Methods in Geometry 1st Edition By Alexander Kharazishvili
$ 25
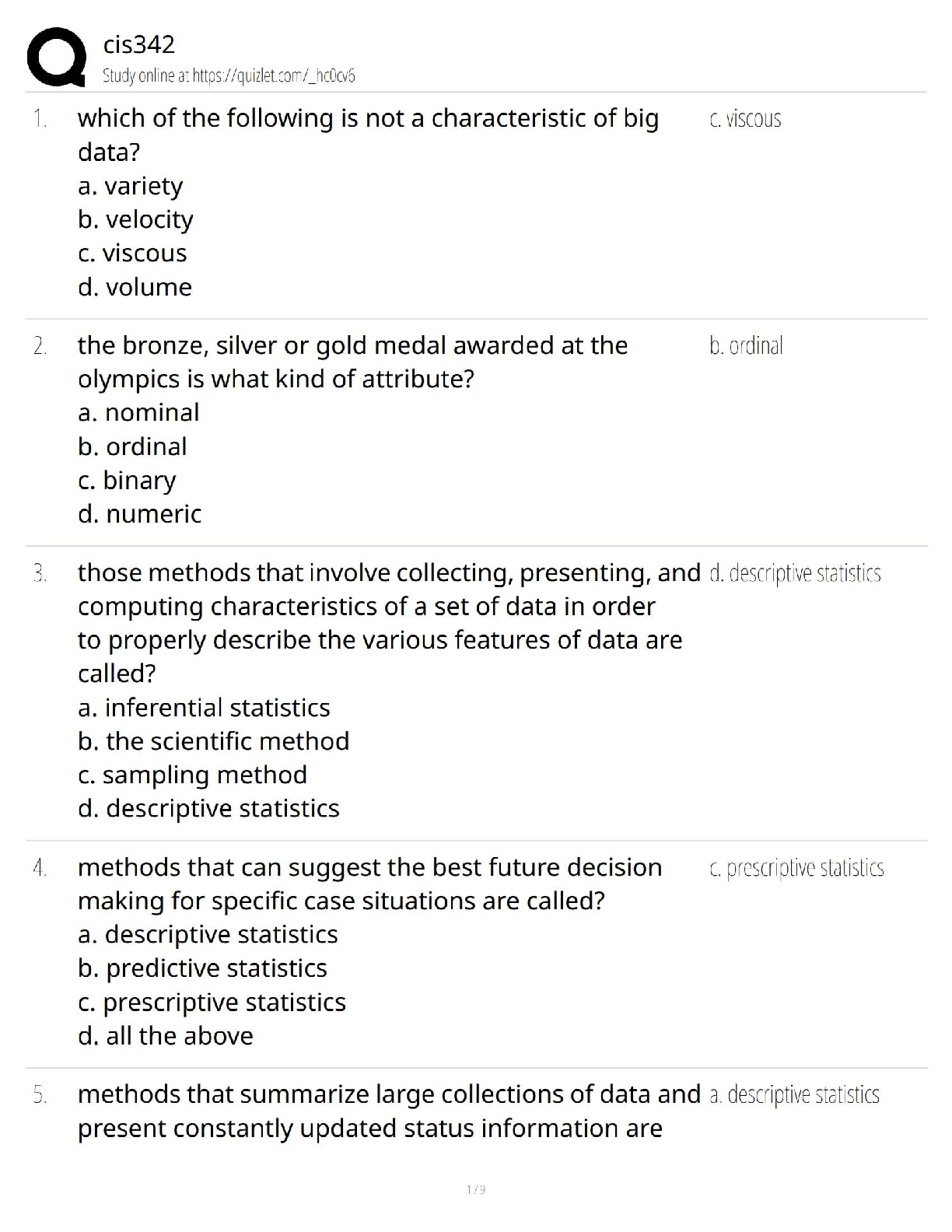
cis342

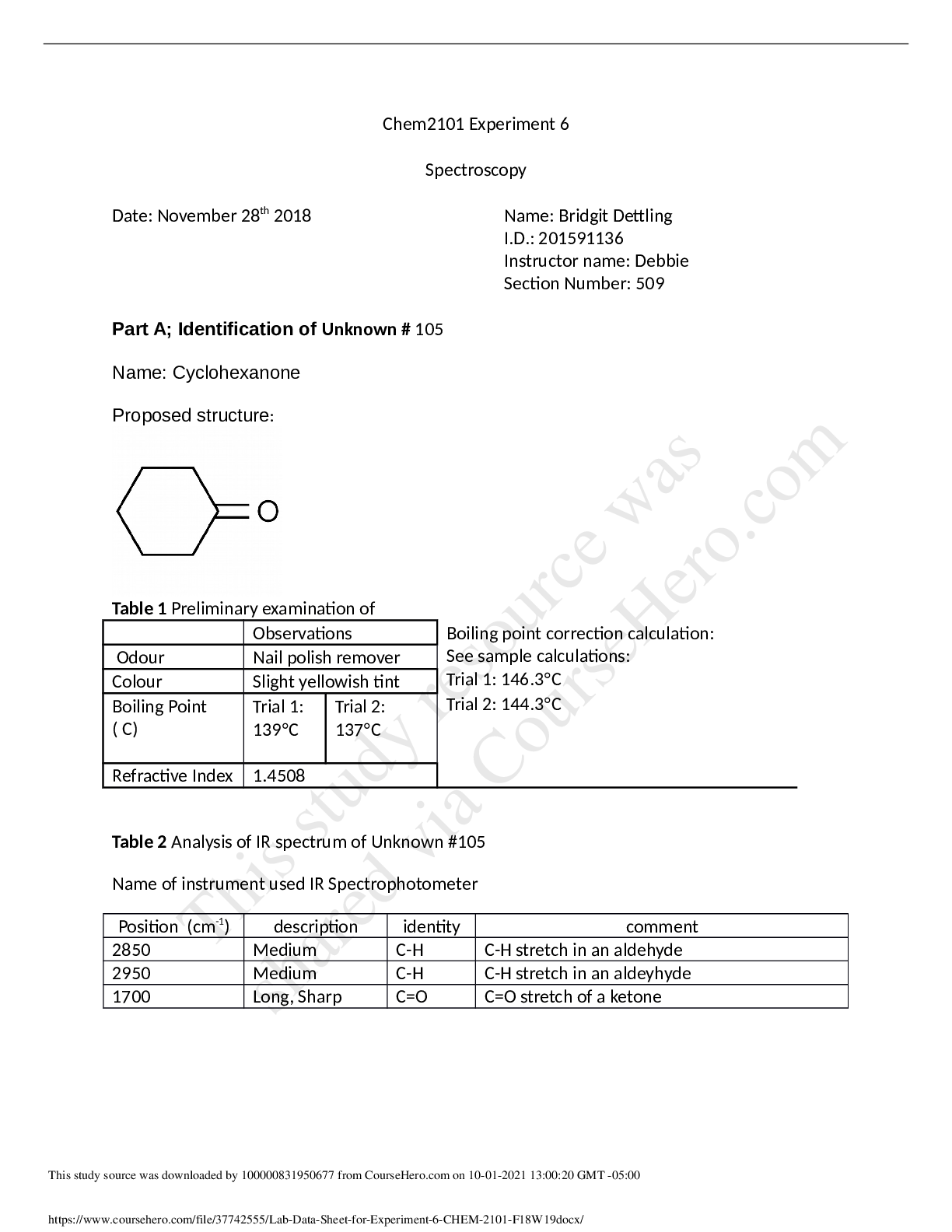
![Preview image of [SOLVED]CHEM 2101 Experiment 5 document](https://www.scholarfriends.com/storage/docx (4).png)


.png)














