PATHOPHYSIOLOGY NR 507WK3TD3
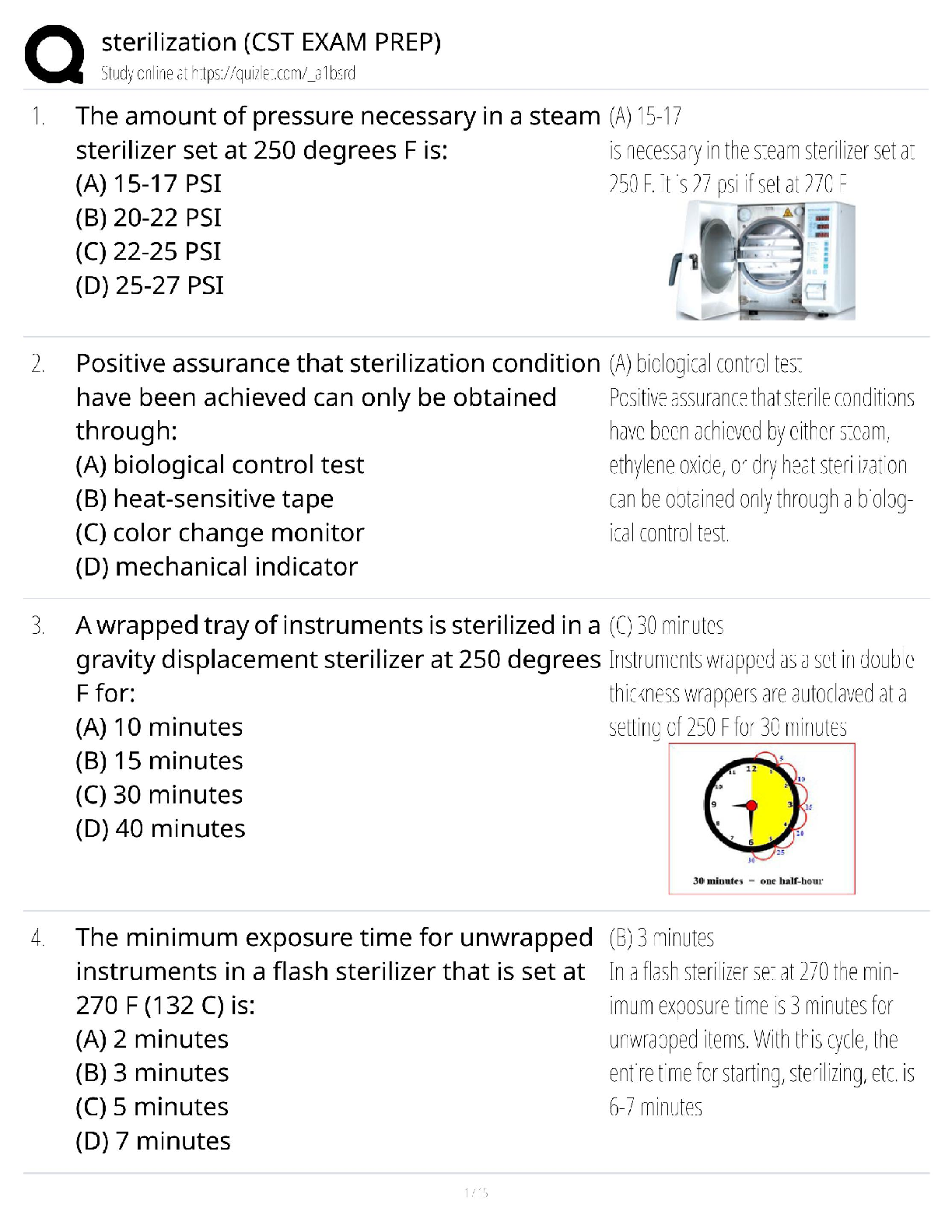
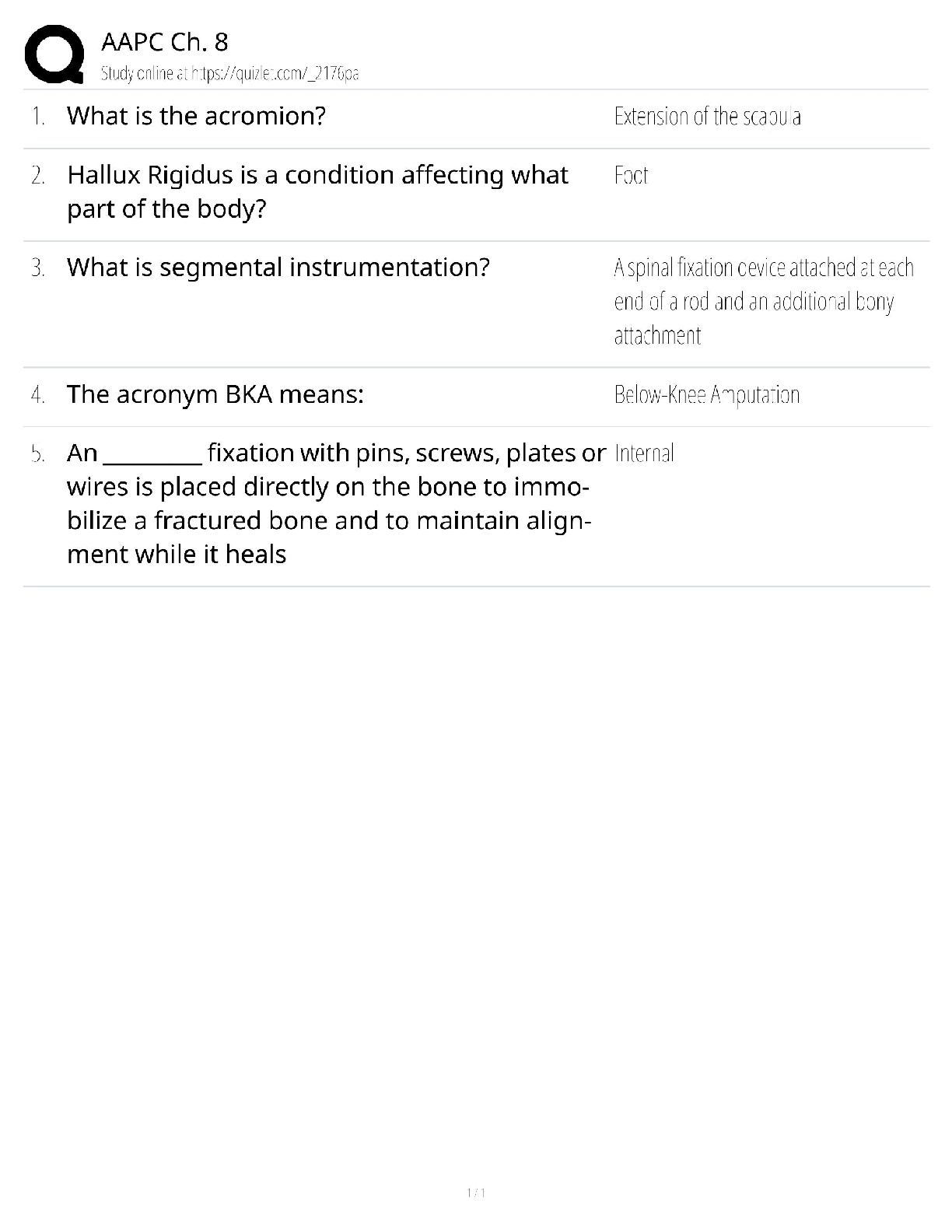
vascular, Cellular, and Hematologic Disorders Discussion Part Three
Loading... Discussion This week's graded topics relate to the following Course Outcomes (COs).
1 2 3 4 5 6 7
Analyze pa
...
PATHOPHYSIOLOGY NR 507WK3TD3
vascular, Cellular, and Hematologic Disorders Discussion Part Three
Loading... Discussion This week's graded topics relate to the following Course Outcomes (COs).
1 2 3 4 5 6 7
Analyze pathophysiologic mechanisms associated with selected disease states. (PO 1)
Differentiate the epidemiology, etiology, developmental considerations, pathogenesis, and clinical and laboratory manifestations of specific disease processes. (PO 1)
Examine the way in which homeostatic, adaptive, and compensatory physiological mechanisms can be supported and/or altered through specific therapeutic interventions. (PO 1, 7)
Distinguish risk factors associated with selected disease states. (PO 1)
Describe outcomes of disruptive or alterations in specific physiologic processes. (PO 1)
Distinguish risk factors associated with selected disease states. (PO 1)
Explore age-specific and developmental alterations in physiologic and disease states. (PO 1, 4)
Discussion Part Three (graded)A new patient is brought into the office for their annual evaluation. The child is a 6-year-old and
appears a bit small for their age but not so small that any alarm bells are set off. The vitals are: P
= 116, R = 22, T = 98.6 , BP = 110/50. (The normal vitals in a 6-year-old are P = 75 – 120, R =
16 – 22, T = 98.6 , BP = (85-115)/(48-64). Examination of the lungs is normal, HEENT is normal,
as is the abdominal exam. The heart however, seems laterally displaced and there appears to be
only a continuous murmur which can be described as crescendo/decrescendo systolic murmur
that extends into diastole. Because, you were trained at Chamberlain College of Nursing you
immediately know that this is probably a patent ductus arteriosus.
F
Explain the murmur from a mechanistic view of the hearts physiological functioning?
What is the epidemiology of a patent ductus arteriosus?
How is a patent ductus arteriosus treated?
Responses
Lorna Durfee
Discussion Part Three
5/18/2016 9:36:07 AM
A new patient is brought into the office for their annual evaluation. The child is a 6-year-
old and appears a bit small for their age but not so small that any alarm bells are set off.
The vitals are: P = 116, R = 22, T = 98.6 , BP = 110/50. (The normal vitals in a 6-year-
old are P = 75 – 120, R = 16 – 22, T = 98.6 , BP = (85-115)/(48-64). Examination of the
lungs is normal, HEENT is normal, as is the abdominal exam. The heart, however, seems
laterally displaced, and there appears to be only a continuous murmur which can be
described as crescendo/decrescendo systolic murmur that extends into diastole. Because
you were trained at Chamberlain College of Nursing, you immediately know that this is
probably a patent ductus arteriosus.
F
Doctor Brown and Class:
Explain the murmur from a mechanistic view of the heart's physiological functioning?
Philips (2015) describes ductus arteriosus (DA) as a vascular connection between the main
pulmonary artery and aorta. When the fetus is developing the DA diverts blood from the
pulmonary artery into the aorta. This diversion bypasses the lungs. When the baby is born,
the DA is supposed to undergo constriction and obliteration. A patent ductus arteriosus
results when the ductus fails to close completely after delivery (Philips, 2016).
The murmur in patent ductus arteriosus is a result of pulmonary vascular resistance that has
fallen and reversal of shunting of blood occurs. The blood shunts left to right from aorta to
the pulmonary artery. This condition increases pulmonary blood flow, and venous return to
the LA and LV and places an increased workload on the left side of the heart. It could also
increase right ventricular pressure if there are pulmonary changes that are vascular that
occur in response to the increased flow of blood and can lead to increased pulmonary. If
pulmonary resistance has fallen, infants will have a continuous-machinery type murmur
that is heard at the left upper sternal border through systole and diastole (McCance,
Huether, Brashers, and Rote, 2014, p. 1203).
What is the epidemiology of a patent ductus arteriosus? Baffa (2014) reveals that
patent ductus arteriosus is responsible for 5 to 10% of heart abnormalities. The ratio of
male to female is 1:3. Patent ductus is seen commonly with the birth of premature infants.
It is present in about 45% of premature infants with a weight less than 1750 g, and it risesto 80% with a birth weight of less than 1200 g. Patent ductus can cause heart failure in
premature infants with a birth weight of less than 1750 gram and 40 to 50% of infants born
with a weight of less than 1500 g (Baffa, 2014).
Patent ductus arteriosus occurs in about 5% to 10% of all congenital defects (McCance,
Huether, Brashers, & Rote, 2014, pp. 1202-1203).
How is a patent ductus arteriosus treated?
Baffa (2014) explains that in the use of Indomethacin in pre-term infants is used with or
without fluid restriction. For term infants or older children, this treatment is not effective.
If a connection persists between the thoracic descending aorta and the pulmonary artery
then surgery or catheter-based correction is indicated (Baffa, 2014). She also informs us
that transcatheter closure has become the treatment of choice in children great than one
year. In those infants, less than one or who have varieties of the ductus, surgery with
division and ligation may be a better choice versus the trans-catheter approach. If the shunt
is large enough and is causing symptoms of heart failure or pulmonary hypertension,
closing it should be done once there is stabilization. For a persistent PDA without heart
failure and pulmonary hypertension closure can be done at any time as an elective (Baffa,
2014).
Lam, Lopushinsky, Ma, Dicke, and Brindle (2015) did a study using meta-analysis
comparing clinical outcomes for surgical ligation or catheter occlusion treatment to see if
one treatment was better. They searched the literature and randomized control trials
between 1950 and 2014 for information. They excluded adult or premature patients and
those with no comparison. They found that both treatments have comparable outcomes,
but re-intervention is more common with catheter-based treatment. Complications appear
lower, and the hospital stay is shorter. They suggest further studies to help determine the
best and optimal treatment (Lam, Lopushinsky, Ma, Dicke, & Brindle, 2015, pp. 784 and
791).
References
Baffa, J. M. (2014). Patent Ductus Arteriosus (PDA). In Merck Manual online.
Retrieved from https://www.merckmanuals.com/professional/pediatrics/congenital-
cardiovascular-anomalies/patent-ductus-arteriosus-pda
Lam, J. Y., Lopushinsky, S. R., Ma, I. W., Dicke, F., & Brindle, M. E. (2015). Treatment
Options for Pediatric Patent Ductus Arteriosus. Chest, 148(3), 784-793.
doi:10.1378/chest.14-2997
McDaniel, N.L. (2014). Alterations of Cardiovascular Function in Children. In
McCance, K. L., Huether, S. E., Brashers, V. L., & Rote, N. S. (Eds.)
Pathophysiology: The biologic basis for disease in adults and children (7th ed.,
pp. 1202, 1203). St. Louis, MO: Mosby.Philips, J. B. (2016). In T. W. Post (Ed.), UpToDate. Pathophysiology, clinical
manifestations, and diagnosis of patent ductus arteriosus in premature infants.
Retrieved from http://www.uptodate.com/contents/pathophysiology-clinical-
manifestations-and-diagnosis-of-patent-ductus-arteriosus-in-premature-infants
5/19/2016 7:58:14 AM
Lorna Durfee reply to Lorna Durfee
RE: Discussion Part Three
Dr. Brown and Class:
Addendum:
My first sentence should say: Baffa (2014) explains that use of Indomethacin in pre-
term infants is used with or without fluid restriction for treatment of PDA.
5/19/2016 7:36:59 AM
Liberty Neoh
Discussion Part Three
Dr. Brown and Class,
Explain the murmur from a mechanistic view of the hearts physiological functioning?
In utero, the role of ductus arteriosus is to shunt the blood from the nonfunctional
fetal lung through its connection between the main pulmonary artery and the proximal
descending aorta. This right-to-left shunt allows the blood with a relatively low oxygen
concentration to be carried from the right ventricle through the descending aorta and
eventually to the placenta, where gas exchange will occur. Immediately after birth, this
blood vessel closes so that the blood can be pumped from the right ventricle to the lungs.
There are occasions when this vessel stays open and this can lead to pulmonary
hypertension and heart failure (Clyman, 2013).
What is the epidemiology of a patent ductus arteriosus?
In the United States, Patent ductus arteriosus is the fifth most common congenital
heart defect (CHD) 5–10% of CHDs. In term infants, the incidence is 0.02–0.006% of live
births; the incidence is increased in premature infants. PDA affects female neonates twice
as often as male neonates. The surgical mortality rate is 20–41% in premature infants with
PDA. In premature infants, spontaneous closure of PDA occurs in 72–75%of cases. If left
untreated, PDA is fatal in 20% of patients by age 20 years, 42% by age 45 years, and 60%
by age 60 years (Kaple & Kornusky, 2016)
How is a patent ductus arteriosus treated?
According to Clyman, Couto, and Murphy (2012), Indomethacin appears to be an
effective alternative to surgical ligation. In most intensive care nurseries, indomethacin and
ibuprofen have replaced surgery as the preferred therapy for closing a persistent PDA.
References
Caple, C. & Kornusky, J. (2016). Patent ductus arteriosus: Management of the neonate.
CINAHL Nursing Guide. Retrieved fromhttp://eds.b.ebscohost.com.proxy.chamberlain.edu:8080/eds/pdfviewer/pdfviewer?
sid=e38f2bde-0b3f-410d-b96e-17990540cd08%40sessionmgr105&vid=11&hid=117
Clyman, R. I. (2013). The role of patent ductus arteriosus and its treatments in the
development of bronchopulmonary dysplasia. Seminars in Perinatology, 37(2).
doi: 10.1053/j.semperi.2013.01.006
Clyman, R., Couto, J. & Murphy, G. M. (2012). Patent ductus arteriosus: are current
neonatal treatment options better or worse than no treatment at all? Seminars in
Perinatology, 36(2). doi: doi: 10.1053/j.semperi.2011.09.022
Instructor Brown reply to Liberty Neoh
RE: Discussion Part Three
5/20/2016 10:28:56 AM
Can you explain the process of pulmonary hypertension? What is going on in the body to
cause this problem?
Liberty Neoh reply to Instructor Brown
RE: Discussion Part Three
5/20/2016 6:14:58 PM
Dr. Brown,
The placenta serves as lungs in utero. Most of the right ventricular output
crosses the ductus arteriosus to the aorta and only 5-10 % of the combined
ventricular output is directed to the pulmonary vascular bed. As the fetus grows, the
pulmonary vascular resistance increases as well. As a result, pulmonary pressures
are equivalent to systemic pressures due to elevated pulmonary vascular resistance.
To facilitate the transition to gas exchange by the lung, cardiopulmonary transition
occurs at birth, characterized by a rapid fall in pulmonary venous return and
pulmonary artery pressure, and a significant rise in pulmonary blood flow. The
most critical signals for these transitional changes are mechanical distension of the
lung, a decrease in carbon dioxide tension, and an increase in oxygen tension in the
lungs. When the normal cardiopulmonary transition fails to occur, pulmonary
hypertension of the newborn will occur. Because a patent foramen ovale and ductus
arteriosus are normally present early in life, elevated pulmonary vascular resistance
in the newborn will produce extrapulmonary shunting of blood, leading to severe
and potentially unresponsive hypoxemia (Puthiyachirakkal& Mhanna, 2013).As a NICU nurse, I have helped caring for babies with this condition.
Recognizing the condition early and providing appropriate management is the key
to prevent long term pulmonary issues. Several randomized controlled trials have
demonstrated the effectiveness of Nitric Oxide which acts as a selective pulmonary
vasodilator. When proper concentration is used, it has been reported that there were
not many side effects (Puthiyachirakkal& Mhanna, 2013).
Reference
Puthiyachirakkal, M.& Mhanna, M. J. (2013). Pathophysiology, management, and
outcome of persistent pulmonary hypertension of the newborn: A clinical review.
Frontiers in Pediatrics, 1. doi: 10.3389/fped.2013.00023
Sarah Boulware reply to Liberty Neoh
RE: Discussion Part Three
Liberty,
The National Health Institute (NIH, 2011) found that medications such as
Indomethacin and Ibuprofen are more effective at closing the patent ductus arteriosus
(PDA) in premature infants. These medications have been found to be less effective in
full term infants. Indomethacin is a non-steroidal anti-inflammatory drug (NSAID) that
inhibits prostaglandin synthesis and facilitates ductal closure. It works by triggering
the PDA to constrict or tighten, which closes the opening. Abdel-Hady, Nasef,
Shabaan, and Nour (2013) found that in preterm infants the ductal tissue matures and
becomes less regulated by prostaglandins with advancing postnatal age. Delaying
pharmacologic treatment decreases the response to the medication, resulting in a lower
success rate. Surgical ligation will be indicated if pharmacologic treatment does not
work. This could explain why the medication is more successful in preterm infants
than full term infants. Full term infants are more resistant to the affects of the
medication because their ductal tissue is already mature and less susceptible to the
stimulation.
Indomethacin can have serious side effects. The medication not only constricts
the ductus arteriosus, it also constricts the arteries that supply blood to the heart, brain,
kidneys and gut. It is responsible for reduced renal, mesenteric, coronary, and cerebral
blood flow as well as decreased cerebral oxygenation. Indomethacin is associated with
transient renal impairment. Indomethacin is the most successful at closing the duct if
given within 24 hours after birth. The infant should be closely monitored for the side
effects associated with the medication (Abdel-Hady et al., 2013).
References
Abdel-Hady, H., Nasef, N., Shabaan, A., & Nour, I. (2013). Patent ductus arteriosus in
preterm infants: do we have the right answers? BioMed Research International. doi:
10.1155/2013/676192
National Institute of Health. (2011). What is patent ductus arteriosus? Retrieved from
5/21/2016 9:56:52 AMhttps://www.nhlbi.nih.gov/health/health-topics/topics/pda
Sarah Boulware
Part 3
5/19/2016 6:42:07 PM
Dr. Brown and Class,
Gehin and Ragsdale (2013) found that patent ductus arteriosus (PDA) is one of the
more common congenital heart defects in children and young adults, occurs twice as often
in girls than boys, and is more prevalent in premature infants than in full-term infants.
According to the National Institute of Health (NIH, 2013), children with parents that have
congenital heart defects are at an increased risk for inherit a heart defect. Maternal disease,
drugs, alcohol, environmental teratogens, and other substances that can interfere with
prenatal development may cause some cases of congenital heart defects. Congenital defects
may be apparent at birth or remain undiagnosed until adulthood. PDA is rarely observed in
adults because it is usually diagnosed in childhood. Since it is often asymptomatic it may
not be detected until a routine screening (Gehin & Ragsdale, 2013).
A heart murmur may be the only sign of PDA. A heart murmur is an extra or un-
expected sound that is heard upon auscultation of the heart (NIH, 2011). The ductus
arteriosus is the blood vessel that connects the aorta and pulmonary artery before birth.
This allows fetal circulation to circumvent the lungs and go directly to the developing
body. This occurs because the fetus receives oxygen and nutrients from the placenta and
umbilical cord, not the lungs. Therefore the respiratory system is not used until the time of
birth. The ductus arteriosus normally closes within minutes to days after birth. PDA occurs
when this duct fails to close and the blood that has already been through the pulmonary
system and is oxygenated and ready to be pumped into the body, back flows through the
opening into the pulmonary artery. This causes oxygenated blood to go back to the lungs
instead of into circulation, forcing the heart to work harder to provide oxygenated blood
into the rest of the body. The left side of the heart usually becomes enlarged. There is a left
to right shunt unless pulmonary pressure begins to exceed systemic pressure. The
continuous crescendo/decrescendo systolic murmur that extends into diastole is a result of
the harsh flow of blood that goes from the aorta directly back into the pulmonary artery
through the unclosed ductus arteriosus upon left ventricular contraction or systole (Gehin &
Ragsdale, 2013).
PDA is treated with medications, catheter-based procedures, and surgery. After
treatment children often go on to lead normal healthy lives (Gehin & Ragsdale, 2013). In
premature infants medication such as Indomehtacin or Ibuprofen is used to help trigger the
opening to constrict or tighten. These drugs do not work in full-term infants. Catheter
procedures are also done to close the defect and reduce the risk of infective endocarditis.
Surgery is indicated to close the defect when the patient is too small for a catheter
procedure or the catheter procedure is unsuccessful. If the patient with PDA has other
congenital heart defects multiple surgeries are usually indicated to correct the defects
(NIH, 2011).
References
Gehin, C. & Ragsdale, L. (2013). Congenital heart defects and medical imaging.
Radiologic Technology, 85(2), 187-210.
National Institute of Health. (2011). What is patent ductus arteriosus? Retrieved from
https://www.nhlbi.nih.gov/health/health-topics/topics/pdaInstructor Brown reply to Sarah Boulware
RE: Part 3
5/20/2016 10:27:55 AM
You mentioned the possibility of infective endocarditis. How would this happen? What is it?
5/21/2016 8:53:24 AM
Sarah Boulware reply to Instructor Brown
RE: Part 3
Dr. Brown,
According to the National Health Insititute (NIH, 2011), in patent ductus
arteriosus (PDA), the increased blood flow can irritate the lining of the pulmonary
artery where the ductus arteriosus connects. This irritation makes it easier for
bacteria that are already in the blood stream to stop and collect in the irritated lining
and colonize, which can lead to infective endocarditis (IE).
Moges et al. (2015) found that IE is an infection of the internal layer of the
heart chambers. It occurs with underlying congenital heart disease and has a high
mortality rate. Early diagnosis and prevention of complications, such as systemic
embolization, are crucial to increasing the chances of survival. Signs and symptoms
may vary but usually include fever and flu like symptoms. Positive blood cultures
with bacteremia often help to diagnose IE. Treatment consists of intense antibiotics,
cardiac surgery, and treatment for systemic embolization.
References
Moges, T., Gedlu, E., Isaakidis, P., Kumar, A., Van Den Berge, R., Khogali, M.,…
Hinderaker, S. (2015). Infective endocarditis in Ethiopian children: a hospital based
review of cases in Addis Ababa. The Pan African Medical Journal, 20(75). doi:
10.11604/pamj.2015.20.75.4696
National Institute of Health. (2011). What is patent ductus arteriosus? Retrieved
from https://www.nhlbi.nih.gov/health/health-topics/topics/pda
Lanre Abawonse
Discussion Part Three
5/19/2016 10:28:29 PM
Explain the murmur from a mechanistic view of the
hearts physiological functioning?
During pregnancy, babies derive their oxygenation from the placenta. With the help
of the ductus arteriosus blood is redirect away from the lungs straight to the body.
After birth and the baby has taken his/her first breath, the ductus arteriosus is no longer
needed. The ductus arteriosus is meant to close after its usefulness has been completed. The
failure to close results in what is known as patent ductus arteriosus. Blood then flows
from the aorta to the pulmonary arteries, causing increased pulmonary vascular pressure.When pulmonary vascular resistance falls, a continuous murmur can be heard (McCance,
Huether, Brashers & Rote, 2013). Etoom and Ratnapalan(2014) noted that a murmur is an
auditory vibration resulting from turbulent blood flow within the cardiovascular system
with varying intensity (loudness), frequency (pitch),, quality, configuration, location(from
high to low pressure areas), and duration.
What is the epidemiology of a patent ductus
arteriosus?
Patent ductus arteriosus (PDA) is the 5th most common congenital
cardiac defect in the US, representing 5–10% of all congenital heart diseases. The
congenital defect is common among infants, with females at a ratio of 2:1 over males. It’s
mostly observed in 8 of 1000 premature live births and 1in 2000 of term live births
(Yzeiraj, Giordano, & Gotfried, 2015). PDA is fatal if left untreated; 20% will die by the
age of 20 years, 42% by the age of 45 years and 60% by the age of 60 years (Schub &
Caple, 2014).
How is a patent ductus arteriosus treated?
The PDA will close by itself 50% of the time, but in the event of lack of
closure, surgery might be required. Another measure is the use of Ibuprofen lysine (IV
preparation): Only for infants 500–1,500 g and ≤32 weeks' gestation: Dose is 10 mg/kg IV
followed by 5 mg/kg 24 and 48 hours later. Indomethacin 0.1–0.2 mg/kg/dose IV q12h for
3 doses can also be used. Ibuprofen lysine is as effective as indomethacin in closing a PDA
and reduces the risk of NEC and transient renal insufficiency (Yzeiraj, Giordano, &
Gotfried, 2015). If these medications fail, surgical ligation is considered (Kang, Samsudin,
Kuruvilla, Dhelaria, Kent, & Kelsall, 2013).
Reference
Etoom, Y., & Ratnapalan, S. (2014). Evaluation of children with heart murmurs. ClinicalPediatrics, 53(2), 111-117. doi:10.1177/0009922813488653
Kang, S., Samsudin, S., Kuruvilla, M., Dhelaria, A., Kent, S., & Kelsall, W. A. (2013).
Outcome of patent ductus arteriosus ligation in premature infants in the East of
England: a prospective cohort study. Cardiology in the Young, 23(5), 711-716.
doi:10.1017/S1047951112001795
McCance, K. L., Huether, S. E., Brashers, V. L., & Rote, N. S. (2013). Pathophysiology:
The biologic basis for disease in adults and children (7th ed.). St. Louis, MO:
Mosby.
Schub, T., & Caple, C. (2014). Patent Ductus Arteriosus: Management of the Neonate.
CINAHL Nursing Guide.
Yzeiraj, E., Giordano, J., & Gotfried, E. (2015) Patent Ductus Arteriosus. In F. J. Domino
(Ed.), The 5-minute clinical consult (Electronic). Philadelphia, PA: Wolters Kluwer
Health.
Instructor Brown reply to Lanre Abawonse
RE: Discussion Part Three
5/20/2016 10:26:39 AM
If the PDA does not close, what patho processes can you expect to see?
Jennifer Roth
Part 3
Hello Dr. Brown and Classmates,
During fetal life, the ductus arteriosus is a normal and essential structure that connects the
pulmonary artery to the distal aortic arch. After birth, with the initiation of pulmonary
blood flow and a right and left ventricle circulation, a variety of physiological and
biochemical signals normally result in complete closure of the ductus (Benitz, 2016). In
patent ductus arteriosus (PDA), a machinery-like murmur can be auscultated at the upper
left sternal border, often radiating down the left side of the sternum into the back. The
murmur is most intense during S2, with a crescendo pattern after S1 and a decrescendo
pattern after S2 (Benitz, 2016). Pulmonary blood flow is excessive and pulmonary
compliance is decreased. “If the pulmonary-to-systemic blood ratio approaches or exceeds
2:1, an apical flow rumble, caused by high flow into the left ventricle, is frequently
present” (Benitz, 2016).
5/20/2016 5:19:56 PMThe incidence of PDA in term newborns is 1 in every 2000 births, representing 5% to 10% of all congenital heart anomalies (Benitz, 2016). It is more common in premature infants, but does occur in full-term infants. PDA is twice as common in girls as in boys.
Most patients with PDA are asymptomatic and do not require any intervention. If there is a larger left-right shunt, there is activity intolerance. A significant PDA that is not closed may lead to issues such as: congestive heart failure, pulmonary hypertension, pulmonary vascular obstructive disease, recurrent chest infection, ductal aneurism and an increased risk of infective endocarditis (Dinarevic, Kadic, Begic, Halimic, & Vukas, 2014). Transcatheter closure is the method of choice for the most children with patent ductus (Dinarevic, Kadic Begic Halimic, & Vukas, 2014). The closure of the ductus is indicated in any child or adolescent with developed symptoms of significant L-R shunt. The PDA may be closed by ligation, where the PDA is manually tied shut, or with intravascular coils or plugs that leads to formation of a thrombus in the PDA (Dinarevic, Kadic, Begic, Halimic, & Vukas, 2014). Fluid restriction and prostaglandin inhibitors such as indomethacin have
[Show More]









.png)

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)