
A Level Geology H414/01: Fundamentals of geology MARK SCHEME - October 2021
$ 5.5

Solutions Manual for Biomolecular Thermodynamics, From Theory to Application, 1e Douglas Barrick (All Chapters)
$ 18

A-level BIOLOGY Paper 1,. 100% EXAMINABLE QUESTIONS
$ 10

eBook [PDF] Comprehensive Textbook of Clinical Radiology, Volume I Principles of Clinical Radiology Multisystem Diseases By Amarnath C, Hemant Patel
$ 30
Shadow Health - Respiratory Lab - Objective Complete (GRADED A+)
$ 7

CSFA Certification Review 9 / Exam Prep 2025 / Score 100% Study Guide & Test Bank / Surgical First Assistant Review
$ 22
.png)
AQA A-LEVEL SOCIOLOGY 7192/3 Paper 3 Crime and deviance with theory and methods Mark scheme June 2021 Version: 1.0 Final Mark Scheme
$ 10

FIREFIGHTER 1 FINAL TEST 2025 || (ACTUAL EXAM SCORED 100%) || STUDY GUIDE FOR FIREFIGHTER 1 FINAL
$ 14.5
NUR 213 Final Exam-with 100% verified answers-2022
$ 12.5

A-level GEOGRAPHY 7037/1 Paper 1 Physical Geography Mark scheme June 2021 Version: 1.0 Final Mark Scheme
$ 7.5

BIO 121 Case Study Module 6_ Essentials in Nutrition
$ 9

AQA A-level CHEMISTRY 7405 Organic Chemistry Mark Scheme. 2021 ASSESSMENT MATERIALS MARK SCHEME
$ 7.5

ABCTE PTK Practice Exam Latest Update Questions And Answers
$ 13.5

eBook Surface Engineering of Biomaterials: Synthesis and Processing Techniques 1st Edition By Ajit Behera , Debasis Nayak, Biswajit Kumar Swain
$ 30

SPēD SFPC: General Questions and Answers Graded A
$ 10

2023 AQA AS ENGLISH LANGUAGE 7701/2 Paper 2 Language varieties Question Paper & Mark scheme (Merged) June 2023 [VERIFIED]
$ 7

>_ GCE Physics B H557/01: Fundamentals of physics || Mark Scheme oct 2021
$ 6

The Bits and Bytes of Computer Networking. Week 2: The Network Layer_ Spring 22/23.
$ 5

Solutions Manual For Calculus Single and Multivariable. 7th Edition By Hallett, McCallum, Lovelock, Osgood
$ 30
EPA 608 Certification Exams with Correct Answers
$ 12.5
.png)
WGU C715 Pre-Assessment Questions and Answers Already Passed
$ 10
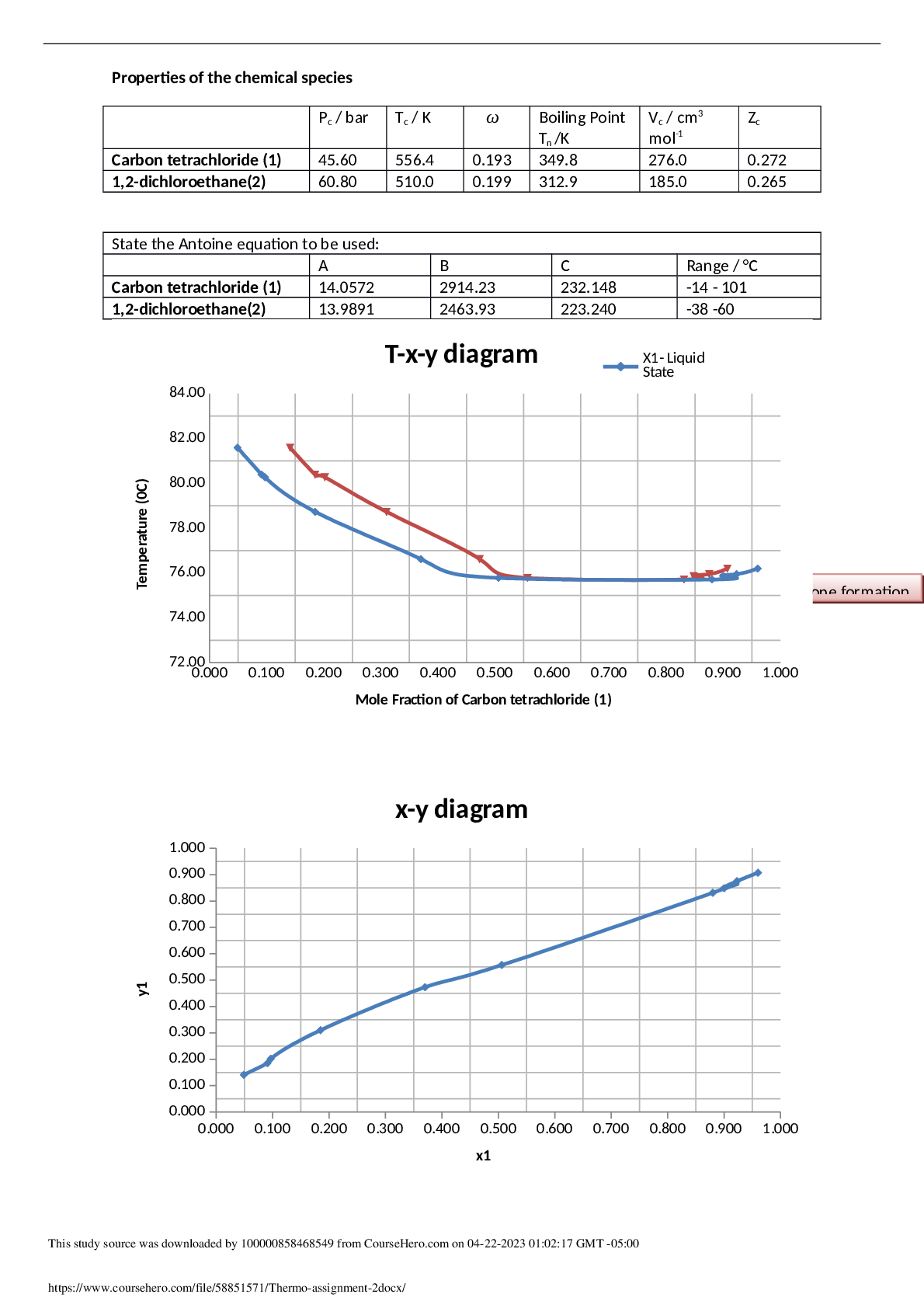
MECHANICS MEE20001 THERMODYNAMICS 1 - Thermo-assignment-2
$ 9.5

BUS 526 NEGOTIATION AND CONFLICT RESOLUTION UNIT 2- CHALLENGE 2 Complete Solution.
$ 9
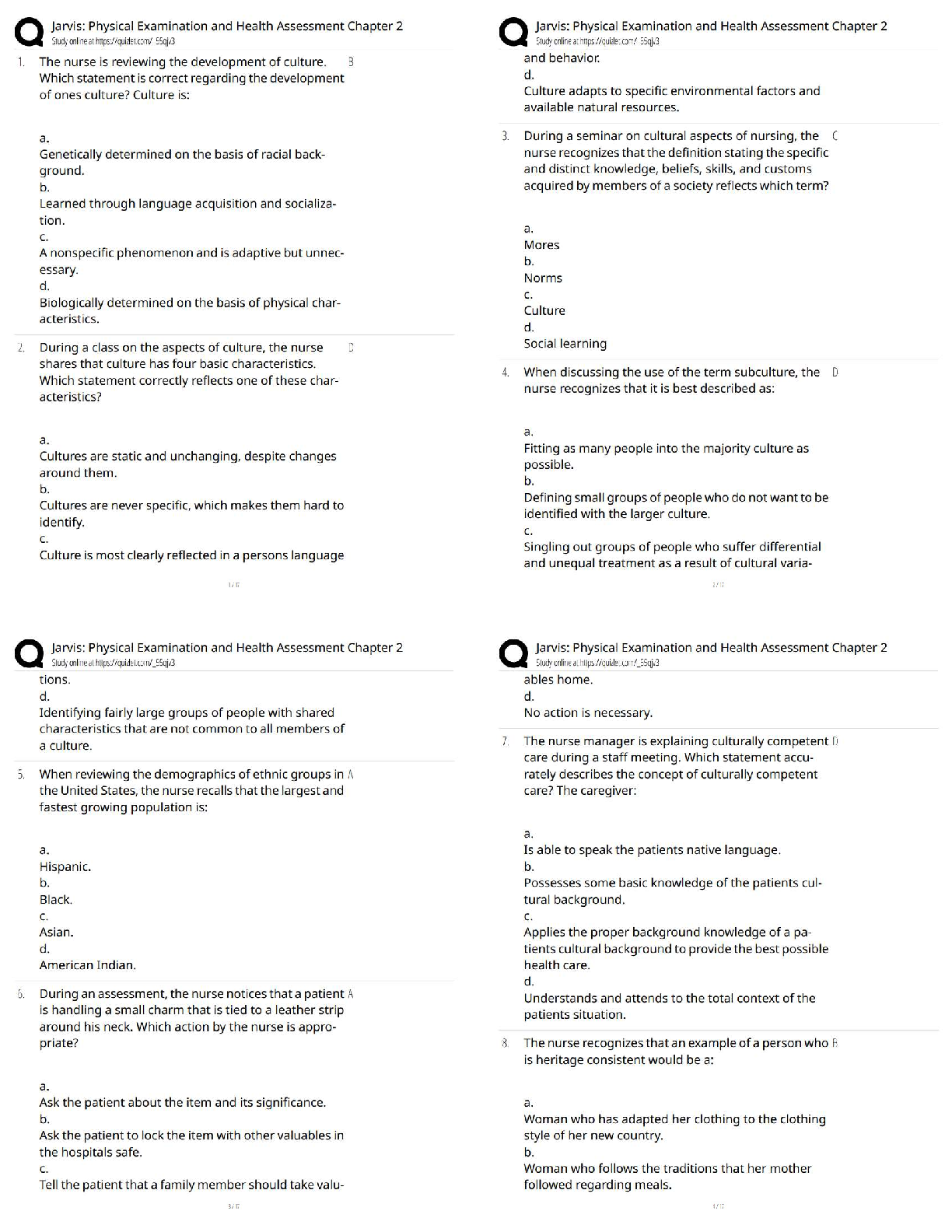
Jarvis Physical Examination & Health Assessment Chapter 2 / Score 100% / New Version 2025 Update / Test Bank & Exam Review
$ 12

FMVA Final Exam
$ 7

NURS 300 Medical Surgical Exam 1| Complete solutions| Excellent Review| Ace Your Exams
$ 10

eBook EPUB PDF International Studies Global Forces, Interactions, and Tensions 3rd Edition By Scott Straus , Barry Driscoll
$ 30
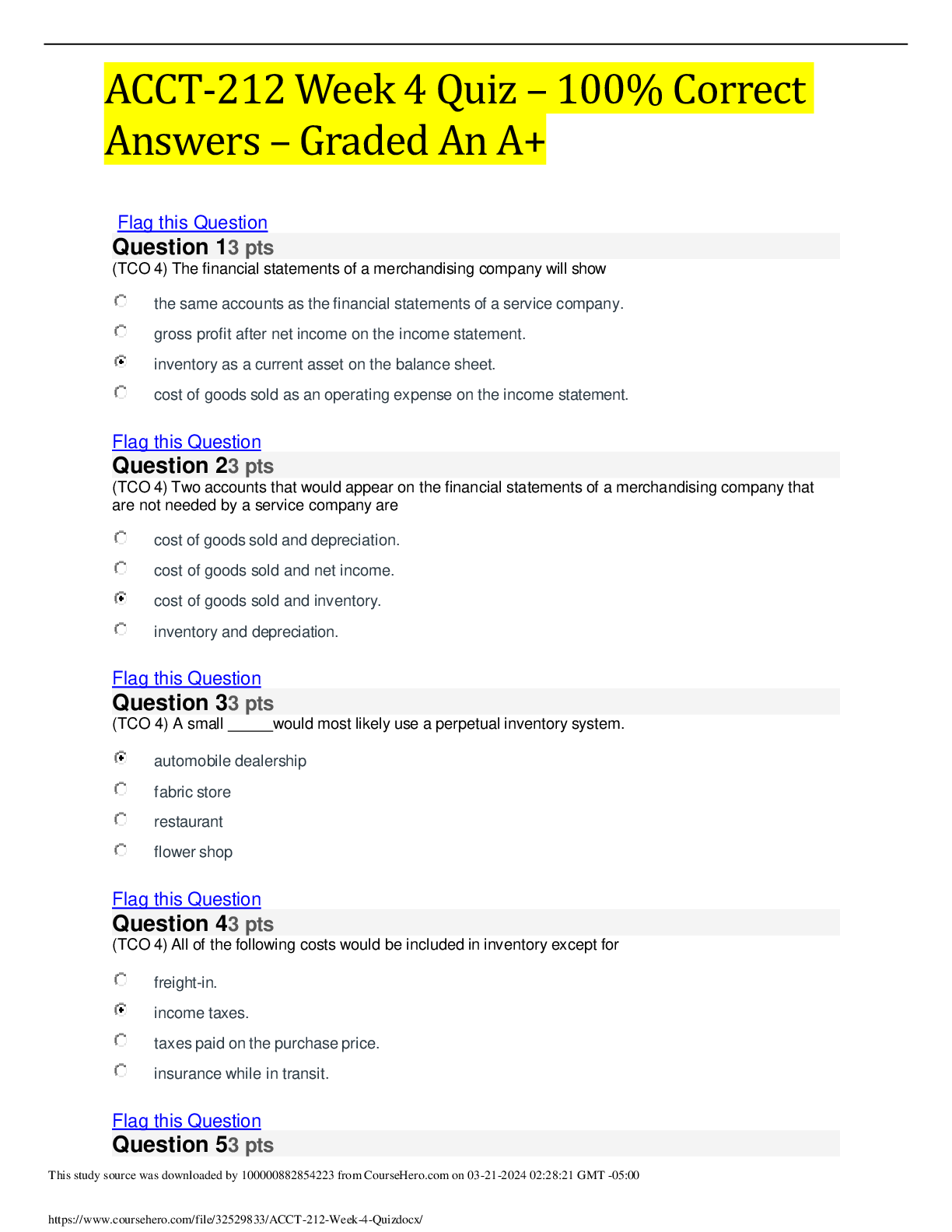
ACCT-212 Week 4 Quiz – 100% Correct Answers – Graded An A+
$ 15

Introduction to Ethics Milestone 1 Adelphi University
$ 18

A Level Latin_H443/02 Question Paper Nov 2020 | Prose Composition or Comprehension



























