Medical Surgical: Kidney Disease
$ 13
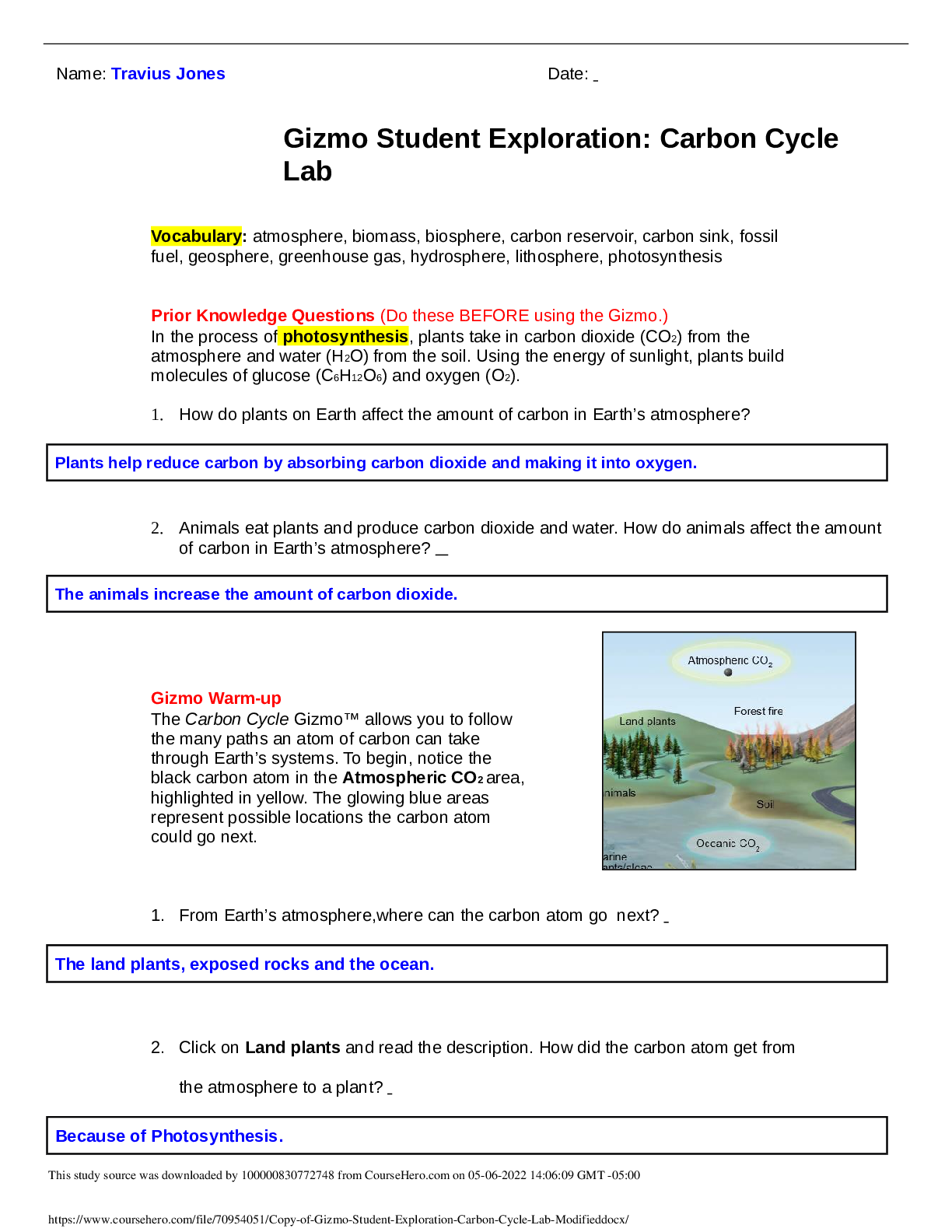
Copy of Gizmo Student Exploration_ Carbon Cycle Lab Modified
$ 7

PEDS 602 COPD Shadow Health Patient: Debbie O'Connor
$ 14

Pearson Edexcel Level 3 GCE English Language Advanced PAPER 1: Language Variation QUESTION PAPER 2022
$ 6

HUMN 303 Week 3 Discussion: Influences of Ancient Architecture (GRADED)