.png)
PHSC 210 Quiz 6 Liberty University Solution; Latest 2020/2021, All Answers Correct
$ 10

MATH1280 Assignment Week 6
$ 15

NURS 6501 Week 3 Quiz : Advanced Pathophysiology -Walden University (Complete Solutions and Resource for the Test)
$ 11

FUTURE SOLDIER TRAINING NEWQUESTIONS AND ANSWERS 2023SOLUTION
$ 11

CHEMISTRY 1601 EXAM 1 PRACTICE QUESTIONS
$ 8.5

Test Bank For Nursing Informatics And The Foundation of Knowledge 4th Edition Mcgonigle
$ 15

CMPS 180: Database Systems I. Textbook: “A First Course in Database Systems”, Jeffrey Ullman and Jennifer Widom, Prentice-Hall, 3rd edition. Science and Engineering Library has textbook on Reserve. 2cd edition is also available, but this course uses 3rd edition
$ 6

eBook [PDF] Ethical Leadership 2nd Edition By Robert McManus, Stanley Ward, Alexandra Perry
$ 30

A&P CLINICAL ASSESSMENT UPDATED 2025 WITH QUESTIONS AND 100% CORRECT ANSWERS GRADED A+
$ 18

WGU D099 OA EXAM TEST BANK 1 /WGU D099 SALES MANAGEMENT OBJECTIVE ASSESSMENT 2026/2027
$ 23
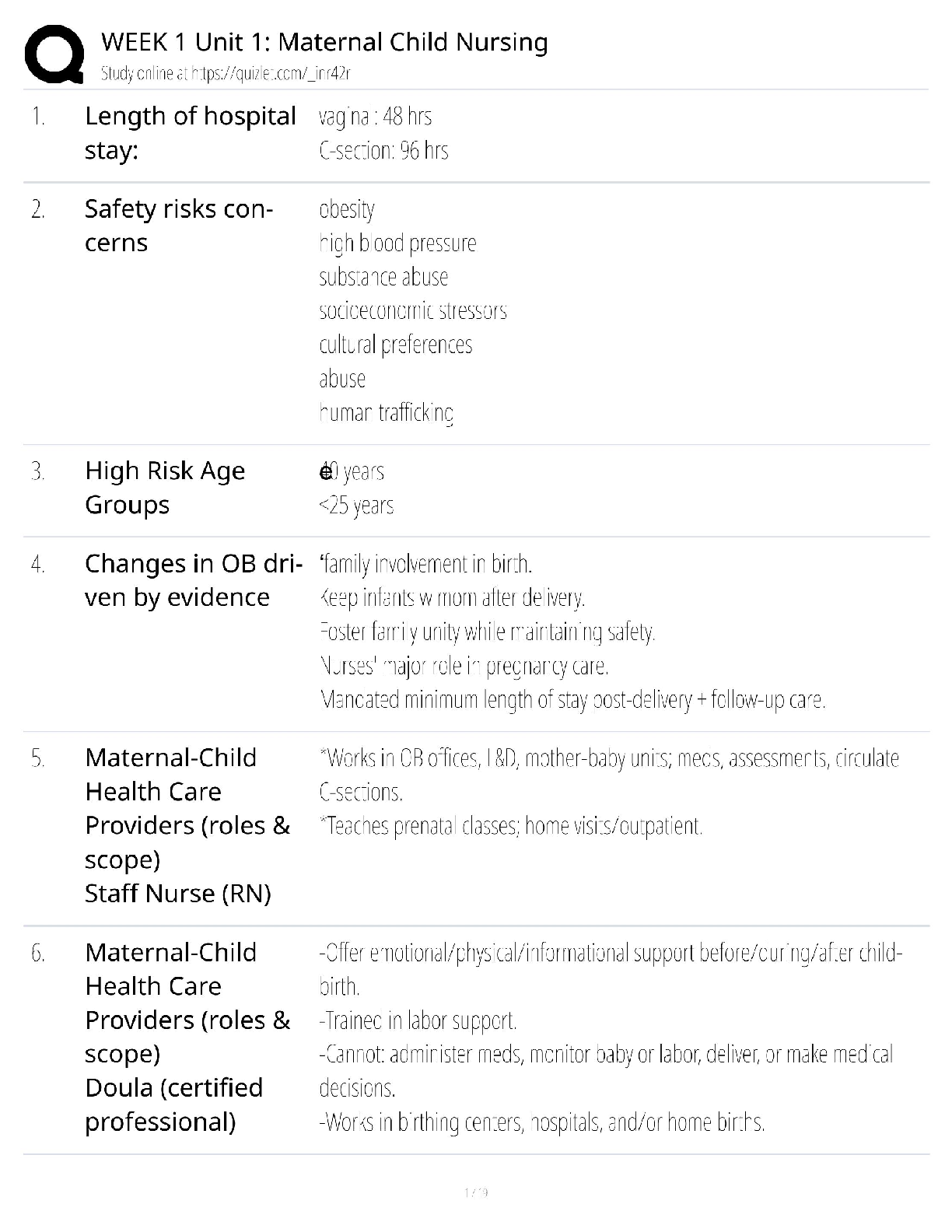
Week 1 Unit 1 Maternal Child Nursing / Score 100% / 2025 New Version
$ 23.5

eBook [PDF] Kafka Connect Build and Run Data Pipelines 1st Edition By Mickael Maison, Kate Stanley
$ 29

C784 Applied Healthcare Statistics | Objective Assessment | Score 100% / New Version / 2025 Update – WGU Exam Prep
$ 14.5

Many of you have experience in complex adaptive systems whether you realize it or not
$ 3.5

Test Bank for Psychiatric Mental Health Nursing Concepts of Care in Evidence-Based Practice 10th Edition Townsend
$ 15

Azure Essentials quiz. Score 100%.
$ 13

CCMA Practice Test 2019 /CCMA Practice Test 2019
$ 6.5

ECON 330 WORKERS AND THE ECONOMY UNIT 2 SUMMARY (Reading and Content) ATHABASCA UNIVERSITY
$ 14

2018_AD_Quizzez.docx
$ 12

APEA- HEME ASSESSMENT EXAM 2024/2025 WITH 100% ACCURATE SOLUTIONS
$ 7

USPS Exam 421 Window Clerk Practice
$ 9

NR-503 Week 2 Discussion: Epidemiological Methods – Rapid HIV Screening (GRADED A)
$ 12.5

Test Bank & Solution Manual for Fundamentals of Corporate Finance, 8th Canadian Edition by Brealey | ISBN 9781260881337 | Complete Chapters 1-26 | Verified Solutions
$ 20

medical-surgical final exam
$ 50.5

BIOL113 Practice
$ 15

2023 GCSE (9-1) English Literature J352/01: Exploring modern and literary heritage texts Question Paper & Mark Scheme (Merged)
$ 7

HESI MENTAL HEALTH RN RANDOM FROM ALL V1-V3 (ALL TOGETHER- VARIOUS TEST QUESTIONS – 38 PAGES OF STUDY NOTE TEST QUESTIONS FROM EXAM)
$ 11

NR 565 Week 6 Readings; Chapter 24
$ 8

OCR A LEVEL JUNE 2022 DESIGN AND TECHNOLOGY PAPER 2 RESOURCE BOOK - FASHION AND TEXTILES.
$ 1

WGU C215 OPERATIONS MANAGEMENT OBJECTIVE ASSESSMENT EXAM LATEST 2024/2025 QUESTIONS AND CORRECT ANSWERS(100% CORRECT VERIFIED ANSWERS)ALREADY GRADED A+
$ 20

Test Bank for Development Across the Life Span 9th Edition By Robert Feldman (All Chapters, 100% Original Verified, A+ Grade)
$ 29
CSCI 2720 Data Structures Math Background Dominance and Order
$ 18

[eBook] [PDF] Middle Range Theories Application to Nursing Research and Practice 5th Edition BY Sandra Peterson, Timothy Bredow
$ 29

SBE.330 Week 3 Case Study, Exploring Innovation in Lighting - Graded An A+
$ 8
, Latest Questions and Answers with Explanations, All Correct Study Guide, Download to Score A.png)
Acute Alterations in Respiratory Function: Technologies, NURS 425 (2021), Latest Questions and Answers with Explanations, All Correct Study Guide, Download to Score A
$ 15

C475 Study Guide 2020
$ 6

Pearson Edexcel As Level 3 GCE 8EC0/01 Economics A Advanced Subsidiary PAPER 1: Introduction to Markets and Market Failure QP June 2022
$ 4

PHARM NCLEX Review. NEW NOTES 2021
$ 10

MISY 5315 Intro to Programming Bus Solutions Quizzes Question
$ 30

BASIC CARE & COMFORT (NURSING) QNS & ANS 20232024
$ 12

NHA CERTIFIED MEDICAL ADMINISTRATIVE ASSISTANT(CMAA) STUDY GUIDE EXAM
$ 12

GCSE PHYSICS 8463/2H Paper 2 Higher Tier Mark scheme June 2020
$ 14
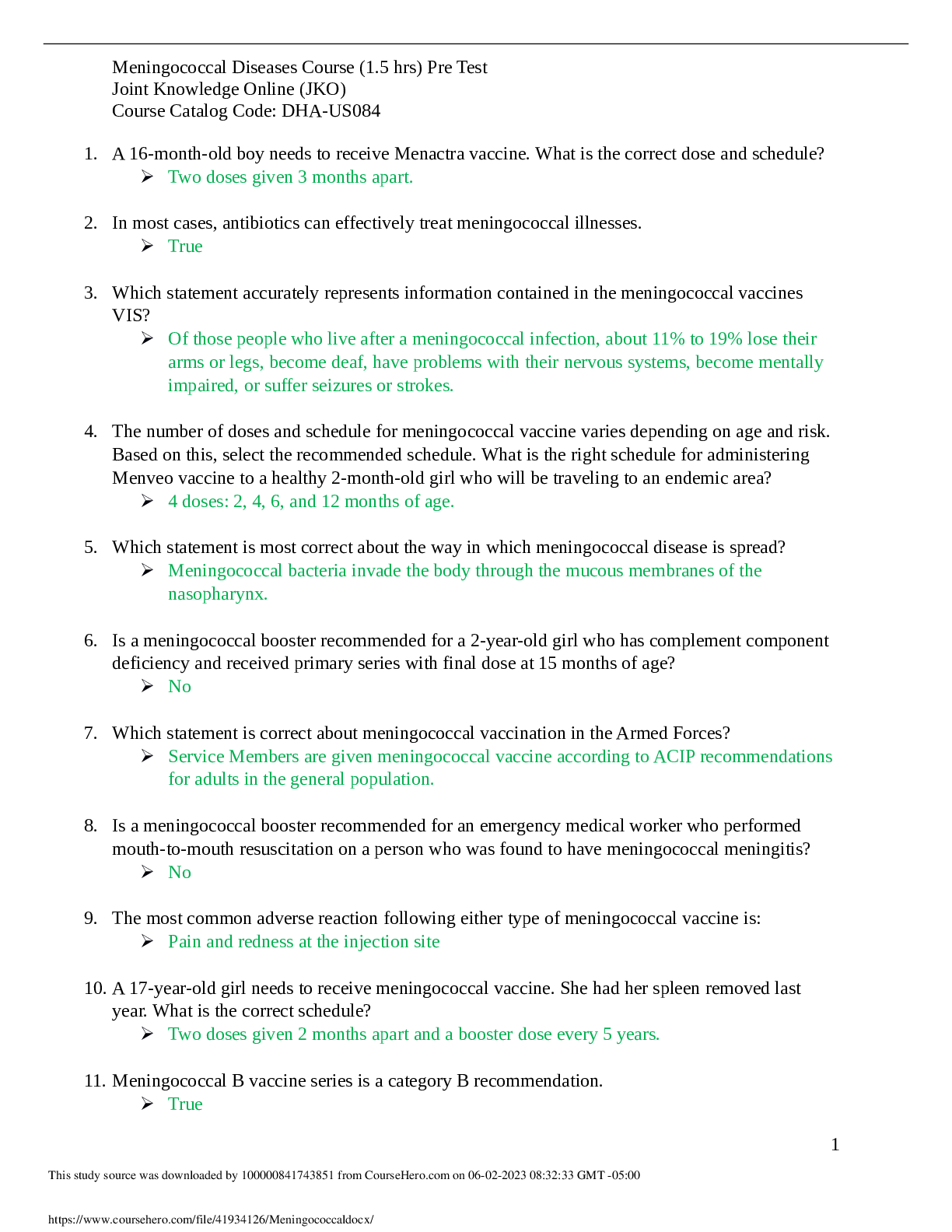
Meningococcal Study Guide 2023
$ 5

CSE 110 All Quizzes | 70 Questions with 100% Correct Answers | Updated & Verified
$ 7

CSIS 330Final Exam questions and answers with all correctly answered 2021
$ 10.5

eBook [PDF] Programming with GitHub Copilot_ Write Better Code–Faster! - Dowswell, Kurt
$ 30

NR 566 wk 5 Study Guide
$ 9.5
.png)
Northwestern UniversityEECS 202HW5_Sol
$ 5

NR509 / NR 509: Advanced Physical Assessment Week 3 Chapter 17 Notes - The Nervous System Latest Update Chamberlain College Of Nursing
$ 7

AQA A Level Physics Paper 1 2019 Question Paper


























